Society
Pursuit of Quality and Trust
Quality Management
Group Quality Management System
At Sysmex, under the supervision and management of the President, the Quality Assurance Department leads our quality management efforts. More specifically, we hold a monthly quality meeting where the managers of our Development, Production, Marketing, and Service Departments explore what we can do to monitor the quality, effectiveness, and safety of our products and services, along with improvement measures. We also hold Quality System Committee meetings regularly to review quality targets, responses to inspections by regulatory bodies, and a management review of instructions for output. This is part of our efforts to maintain the Group’s quality management system and promote activities for improvement.
All our production facilities for final products* have obtained ISO 9001 or ISO 13485 certifications. Of the 83 companies in the Sysmex Group, 35 have been ISO 9001 certified and 21 have been ISO 13485 certified. In fiscal 2024, three cases of nonconformity were identified in an internal quality audit and two cases during an external quality audit. Remedial action is being taken. In addition, we have been making efforts to improve quality by setting the numbers of recalls and FDA warning letters as indicators for monitoring the progress of sustainability targets.
- Wholly owned subsidiaries
Sustainable Improvement Programs
Compliance with Related Laws and Regulations in Each Country
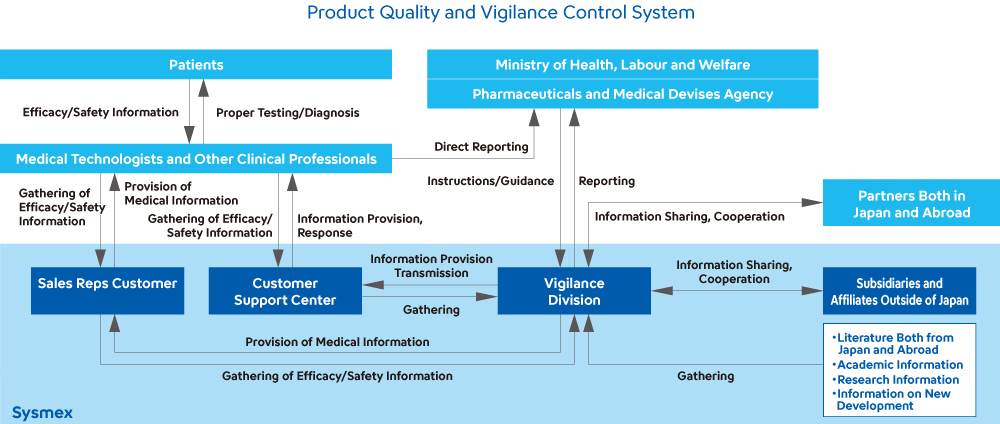
Used in laboratory testing, Sysmex’s products play a vital role in protecting human life and health. Sysmex has created a system that allows us to thoroughly comply with regulations worldwide, including the Japanese Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices, the In Vitro Diagnostic Medical Devices Regulation in the EU, the U.S. FDA’s Quality System Regulation, and the Regulations on the Supervision and Administration of Medical Devices in China, as well as maintaining and improving the safety and quality of our products.
Reinforcing Structures for Maintaining and Enhancing Quality
In the product development process leading up to market launch, Sysmex verifies product quality by setting up five "quality gates."1 We also conduct quality and safety-related risk assessments when designing and developing new products, as well as when changes are made to the designs of existing products. If a high-risk event is noted, we act to eliminate it. In addition, when marketing products manufactured by other companies, we verify their quality by conducting audits of the manufacturers and meticulously inspecting their products. Moreover, in the unlikely event that a product defect occurs, we have systems in place to quickly identify the details and respond to any problems.
At factory sites, we conduct regular quality audits and monthly monitoring of manufacturing processes and supplier conformity to ensure quality. We appropriately instruct and support suppliers with high nonconformity rates to improve their quality. Our global quality complaint processing system allows us to gather quality information from markets around the world in a timely manner. When we receive information about a problem or malfunction, we immediately investigate the cause and cease distributing the product in question. If we need to take any corrective or preventive actions regarding a problem or malfunction, we promptly plan such actions in accordance with the Group’s regulations, carry out the plan, and later verify the validity and effectiveness of the actions taken.
To ensure quality and traceability, we use RFID2, GPS and temperature data loggers3 when transporting certain reagents in testing, enabling high-level quality assurance for products that require stringent temperature control.
- 1 Product design assessment, process design assessment, evaluation by the Product Quality Control Department, quality management system (QMS) checks during the manufacturing process, and inspections for mass-produced items
- 2 A system that uses radio waves to read and write data on RF tags without physical contact. The RFID tags used are passive type, which do not emit radio waves themselves but receive signals from an RFID reader to allow non-contact and batch reading of information.
- 3 A measurement device equipped with a thermometer and a data logger
Providing High Quality Products and Services through Third-Party Certification
To enhance its credibility, Sysmex is strengthening its quality assurance system regarding product inspection results.
Our Ono Factory is ISO 17034 certified. This international standard relates to the competence of reference material producers. This was the first such certification granted in the hematology field in Japan. It recognizes a manufacturer’s ability to provide reference materials of the proper quality. This certification strengthens our credibility concerning the quality of data of our products and services, enabling our customers in global clinical laboratories to verify their own competence to provide proper test data.
Employee Training
Focusing on Specialized Quality and Safety Training
In addition to Quality Policy training, Sysmex provides regular quality management training to the relevant departments, as well as specialized training on laws and regulations for employees in specific departments or job categories. In fiscal 2024, we provided training on the topic of quality to a total of about 2,800 employees across Japan in the Group companies’ various development, production, and marketing and service departments, as well as in ISO-certified business offices. We also held training regarding quality at all production facilities for final products and ISO-certified business offices managed by our Group companies overseas.
Management of Information Regarding Quality and Safety
Sharing Customer Feedback within the Group
Sysmex established the Quality Assurance Department, which controls information regarding the quality and safety of our products. Its function is to handle inquiries it receives from outside the Company, as well as to investigate and analyze the information it receives; to share this information with the Design, Manufacturing, and other divisions, and to improve quality. In addition, we have established a structure for incorporating this information in the next generation of products.
Product Recall and Repair Information Posted on Our Website
Sysmex posts information about product recalls and repairs on its website under “Important Product Notices.”
Response to the Circulation of Counterfeit Reagents
To assure accurate testing results, Sysmex asks its customers to use Sysmex-branded instruments and reagents together. In recent years, however, counterfeit Sysmex reagents have been found to be in circulation in some instances. The use of such counterfeit reagents endangers the reliability of testing results, and in some cases, they can be harmful to patients’ health. For this reason, Sysmex continuously monitors markets for counterfeit reagents. When they are discovered, we exercise our intellectual property rights and work with local government institutions and judicial bodies to ensure swift and thorough responses.
Enhancing Customer Satisfaction
High Marks in Customer Satisfaction Surveys
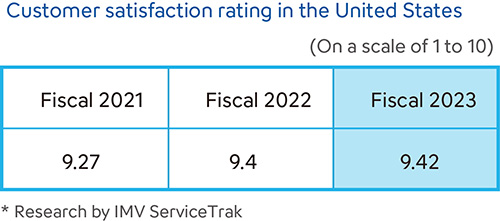
Sysmex conducts customer satisfaction surveys in various countries and regions. Each country or region uses its own metrics based on the products and services offered in order to provide enhanced service and support. In Japan, we conduct periodic customer satisfaction surveys and disclose the results. Sysmex America has earned the No. 1 ranking for 18 consecutive years in the hematology analyzer manufacturer category of the IMV ServiceTrakTM organized by IMV, a specialist provider of market research to the medical imaging and the clinical diagnostic instruments fields. The company also earned top ratings in 91% of the service-related categories and has received all three IMV awards—Customer Satisfaction, Service, and System Performance—for seven consecutive years.
We’re using our own surveys and third-party evaluations to regularly monitor whether we are providing high-quality products and services that satisfy our customers.

Stakeholder’s Voice
On the Front Lines of U.S. Clinical Laboratories: Solving Issues Through Service and Support

Senior Executive Officer, Customer Care / Sysmex America, Inc.
I always tell the Customer Care team, “Every sample is not only a test; it’s a patient. Imagine that your loved one is at the other end of the service you provide and always do your best to solve our customers’ challenges.” I am convinced that Sysmex has been able to build an unwavering service and support brand because our strong sense of purpose—to contribute to patient health—has deeply embedded itself in our corporate culture, especially among the clinical laboratories that are our customers.
Click here for details:
On the Front Lines of U.S. Clinical Laboratories: Solving Issues Through Service and Support
Efforts to Incorporate Customer Feedback in Our Products and Services
Requests and comments from customers are gathered by Sysmex’s Voice of the Customer (VOC) Team. After analyzing this information from various perspectives, the results are provided as feedback to related divisions in order to utilize them in the development of new products and in operational improvements. In fiscal 2024, we gathered approximately 15,000 customer feedback responses from the Japanese market and many from Europe and other foreign markets. We have received a positive response to instruments with new functions and equipment that we have added as a result of customer input, which is indicated by the VOC mark in our product catalog.

Providing Highly Satisfying User Training
Sysmex provides a variety of training programs as part of our customer support, including instrument operation, maintenance, and application support, using a globally consistent digital platform called CaresphereTM Academy. In recent years, we have established new training centers in Brazil and Turkey, with the aim of strengthening and enhancing customer care by offering high-quality face-to-face training tailored to local needs.
We are also developing and expanding access to online training environments in regions around the world. In addition to e-learning, which enables customers to develop skills anytime and anywhere, we also offer highly immersive virtual training through full-scale online studios. This enables customers in remote areas to receive standardized, high-quality product training, even if they are far from a training center.
-

Training Center in Brazil -

Training Center in Turkey
Evolving Customer Care Activities through the Use of Advanced Digital Technologies and Information Assets
Sysmex pioneered the introduction of network solutions in the industry introduce network solutions that connect testing instruments to a network and remotely monitor their operating status for predictive maintenance purposes. Today, a global team of experts regularly monitors data collected from products provided in more than 190 countries and regions worldwide, including instrument failure status and service conditions, to help improve quality, speed up service improvement processes, and reduce instrument failure rate.
To maintain quality and speed in services, we have revamped our market support escalation system. This new system allows customer issues and inquiries from any region of the world to be escalated to our headquarters along with detailed information, facilitating faster problem resolution. We are also developing an online application to support all service activities by visualizing instrument log data and assessing instrument status using AI models.
By combining accumulated digital information assets with advanced technologies such as AI, we are evolving toward a more proactive service model.
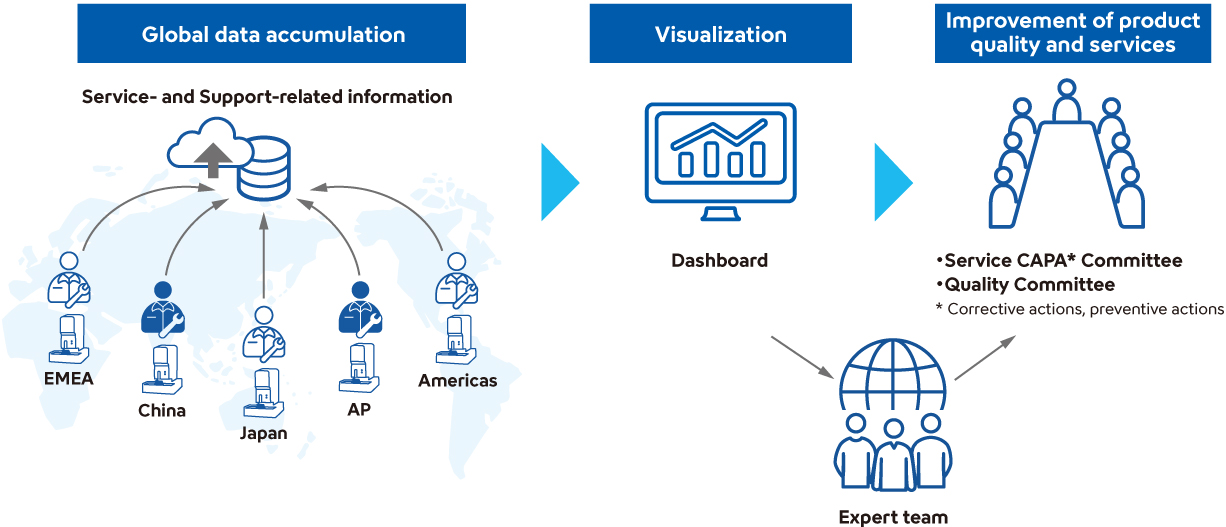
Strengthening Customer Care Functions Through Global Knowledge Integration and Sharing
In 2024, Sysmex launched a system to integrate and share knowledge—expertise, experience, skills, and know-how—acquired through service and support activities across the Sysmex Group worldwide. Knowledge gained through frontline customer care activities is applied to provide high-quality and efficient customer support that does not rely on individual personnel, regions, or levels of experience. Furthermore, by centrally aggregating various resources—such as product information, case studies of inquiries, and academic literature—we are enhancing the academic knowledge of application support staff and improving the efficiency of customer support delivery.
Stakeholder’s Voice
The Backbone of Our Global Leadership: How Sysmex Brings Value to Medical Institutions with Service and Support

Michiko Yoshimoto, Vice President of Application Support, Global Management Division
Hematology, in which we hold the world’s top market share, plays a fundamental role in disease screening. Despite daily quality assessment, unexpected test results may arise due to patient-specific factors. In such cases, we ensure the reliability of test results by providing prompt support and working closely with the customer to identify the cause and interpret the data.
Click here for details:
The Backbone of Our Global Leadership: How Sysmex Brings Value to Medical Institutions with Service and Support
Disseminating Useful Information
Sysmex Corporation disseminates valuable information to its customers in Japan through the Support Information section of its website. Sysmex continually expands the functionalities of the website to ensure that customers have the ability to use the website to a greater extent. Examples include adding an email magazine distribution service, which communicates the latest information, and "My Page" functionality, which allows users to manage content and their browsing histories.
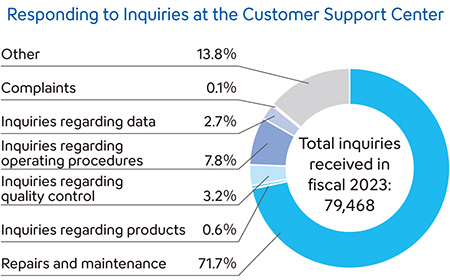
Responding to Inquiries Rapidly and Responsibly
The Sysmex Group has established regional customer support centers to reply quickly and carefully to inquiries, as well as to respond promptly to customer requests.
In Japan, we have established a Customer Support Center, where experienced staff members with expert knowledge respond to customer inquiries 24 hours a day, 365 days a year (separate agreement required for use). By creating a database containing maintenance histories and the details of past inquiries from customers for quick reference, the center responds to inquiries and requests rapidly and carefully.
Scientific Activities
Sysmex holds scientific seminars to impart the latest information about clinical testing in various countries and regions across the world. In Asia, we work with government agencies, including national health ministries and major academic societies, to conduct scientific activities designed to help improve the quality of clinical testing.
Holding Scientific Seminars for Medical Professionals

Sysmex has held annual Scientific Seminars since 1978 to discuss topics selected from a wide range of medical research areas and to provide opportunities to share knowledge from the latest information and research. The topic in 2024 was “Future Perspectives in Cardiovascular Disease Research,” and the seminar was held onsite and online in a hybrid format. The lectures in Kobe and Tokyo were streamed not only domestically but also internationally. The seminar was conducted primarily in English, with simultaneous interpretation offered in Chinese, Indonesian, Thai, and Vietnamese. We were pleased to be able to welcome participants from 21 countries worldwide. After the seminar, videos of the lectures were distributed globally. We also conducted country-specific participant surveys to identify medical issues and interests that differed depending on country and region. We utilize such information for future seminar theme setting and the Group’s initiatives.
In addition, we have held many other seminars globally with a wide range of attendees. Through these initiatives, we aim to build trust-based relationships with medical professionals and help improve healthcare quality globally.
Activities for Patients and the General Public
Sysmex set up the online scientific information website “Medical meets Technology” to provide information on the varied roles of technology in healthcare from a scientific viewpoint in an easy-to-understand format.
In addition, from the viewpoint of informed consent, product information for the NCC Oncopanel™, a testing system for cancer genome profiling, has been newly developed with easy-to-understand explanations for patients and their families. Such information was previously only available to medical professionals. In response to issues related to antimicrobial resistance (AMR), we are conducting a variety of initiatives, which include the global implementation of “#AMRfighter”, an awareness-raising activity, and widely distribute a scientific booklet titled “Proper Use of Antibiotics” directed at patients and the general public.