Society
Improvements in Accessibility to Healthcare - Approaches to Global Health and Universal Health Coverage -
Today, many global health issues affect health worldwide and require international collaboration to be solved. Many of these issues are threatening the health of people who cannot access proper medical care due to inadequate healthcare environments and systems.
In the field of global health, Sysmex works to solve issues in testing and diagnosis, the core of its business. As one of our responsibilities as a global company, we will contribute to Universal Health Coverage (UHC)* by promoting quality testing in emerging and developing countries so that as many people as possible can receive appropriate medical care.
- UHC: A condition in which all people have access to affordable and proper services to improve their health, prevent or treat illnesses, and recover.
Contribution to Malaria Elimination
Transmitted by mosquitoes, malaria is one of three major infectious diseases defined by the World Health Organization (WHO) and is prevalent mainly in tropical and subtropical regions. As blood samples are used in testing for malaria, applying technology accumulated in the hematology area, Sysmex developed an automated hematology analyzer to support standardization and optimization of malaria testing. The number of deaths caused by malaria can be reduced through early detection and treatment. By providing an instrument for use in clinical settings that can swiftly and conveniently produce useful results for diagnosis, Sysmex is contributing to the elimination of malaria.
Realizing High-Precision and Simple Malaria Testing
The current mainstream method of testing for malaria uses a rapid diagnostic kit or a microscope. However, both options pose problems such as the time required, ranging from 15 to 30 minutes, including pretreatment, and the requirement for skilled techniques in microscopic testing. In contrast, our hematology analyzer identifies red blood cells infected with the malaria parasite and determines the percentage of infected cells without pretreatment, automatically performing both processes in about one minute1 with a high degree of accuracy.2 In addition, since our hematology analyzer calculates eight CBC parameters3 that are measured at the same time in normal hematology testing, it can provide clinicians with data on other issues, such as anemia and nutrition status, in addition to detecting malaria. Through the use of this technology and product, Sysmex is supporting clinical settings in areas where malaria is endemic.
Since 2016, Sysmex Corporation has been involved in the initiatives of the Malaria Consortium, which consists of research institutes and enterprises combating malaria, and contribute to project activities in the field of testing and diagnosis conducted through industry-government-academia partnerships in Asia and Africa.
At the 11th Nikkei FT Communicable Disease Conference held in October 2024, Sysmex introduced its activities in malaria-endemic regions and expressed expectations for information dissemination at the Ninth Tokyo International Conference on African Development (TICAD9).
- 1 Time from the start of measurement to the determination of test results.
- 2 Testing by the analyzer does not mean that malaria diagnosis will replace microscopic testing. Nor does it mean a diagnosis can be made based on the outcome of analyzer testing alone. Diagnostic confirmation is based on a doctor’s comprehensive judgment, which includes other clinical information.
- 3 Red blood cell count (RBC), white blood cell count (WBC), hemoglobin volume (Hb), hematocrit value (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count (PLT).

Stakeholder’s Voice
Interview with Medical Professionals in Kenya
Sysmex’s analyzers are extremely valuable. A patient’s blood sample had been diagnosed as malaria-negative using both the smear method and a rapid diagnostic kit. However, the physicians still suspected malaria and sent the sample to us for a more accurate diagnosis. When measured using Sysmex’s analyzer, we were able to detect a very small number of malaria parasites, confirming the diagnostic superiority of the device. The analyzer provides an accurate diagnosis in less than one minute after setting the sample, and it precisely measures the number of parasites in just one microliter of blood, including even minute proportions. Investment in such advanced diagnostic technologies is highly valuable in significantly enhancing patient management.

11th Zero Malaria Award Received

Supporting HIV Countermeasures in Emerging and Developing Countries Through a WHO Prequalified Testing System

In emerging and developing countries, Sysmex Partec provides a testing system for CD4+ lymphocytes to assist in diagnosing HIV-infected individuals and monitoring their immunological status. It has provided approximately 30 million tests since 2011 on a cumulative basis. The system measures the number and proportion of CD4+ lymphocytes in the blood in just three minutes and is inexpensive, small, and portable, requiring only simple maintenance. Through these features, it aims to ensure everyone has equal access to simple, rapid, and stable testing.
In addition, this system has acquired prequalification* by the WHO and has been promoted for introduction in countries and regions in which medical resources are limited. It improves the quality of HIV diagnosis and treatment in emerging and developing countries.
- Certification system under which the WHO guarantees quality, safety and efficacy, with the aim of ensuring that health care products, including pharmaceuticals, testing, and vaccines, can be used with a sense of security in countries lacking in resources. The system was launched in 2001 for pharmaceuticals for HIV/AIDS and is now used as a reference list for procurement in emerging and developing countries. The Global Fund to Fight AIDS, Tuberculosis, Malaria, and other funding organizations preferentially choose products that have acquired prequalification.
Contribution to Strengthening Healthcare Systems
Emerging and developing countries need to develop the abilities of medical professionals (capacity building) to solve health and medical issues. Sysmex continuously provides products, services, and support to medical institutions while increasing opportunities to provide training and scientific information to medical professionals. It contributes to the early detection and treatment of diseases, as well as improving diagnoses and treatment methods by emphasizing the significance and clinical value of testing and the dissemination of diagnostic technology.
Training for Medical Professionals
Sysmex has established an in-house training center called Sysmex Academy. In addition, it provides globally unified educational content and skill management tools through the Caresphere™ Academy for online training. This enables us to conduct educational programs on clinical value and training sessions on instrument maintenance for sales distributors and medical professionals. In Africa, Sysmex offers mentorship training that it developed to ensure that laboratories’ quality management systems conform to the international ISO 15189 standard.
Initiative for Digital Transformation (DX) in Ghana

In fiscal 2023, Sysmex Corporation introduced an application (Caresphere™ XQC) for real-time external quality assessment in clinical laboratories on a trial basis in Ghana through the Ministry of Economy, Trade and Industry's “Feasibility Study Project on Emerging Business Opportunities in the African Market (AfDX),” to investigate local needs and issues related to the diffusion of services. Training on Caresphere™ XQC was provided to six medical facilities participating in the trial, allowing them to experience firsthand the value of the service offered. Seminars and other activities were also held to promote understanding of quality improvement in clinical testing among local governments, industry associations, and medical institutions.
By leveraging not only digital solutions but also Sysmex’s expertise in supporting the spread of external quality assessments in Asian countries over the years, and the public-private partnership scheme for this project, we will contribute to the improvement of local medical standards by promoting the understanding of external quality assessments, and by developing human resources through operational training provided to the Ghanaian Ministry of Health, as well as medical institutions in Ghana.
Supporting Quality Control and Standardization of Clinical Testing in Asian Countries

Sysmex has been engaged in support activities to improve the quality and accuracy of clinical tests in Asian countries.
In Mongolia, under the cooperation of the Mongolia’s Ministry of Health, Sysmex continues to support five testing fields (hematology, clinical chemistry, immunochemistry, hemostasis, and urinalysis). For facilities in urban areas that face difficulties participating in external quality assessment (EQA) programs, Sysmex provides technical and academic expertise to enable EQA implementation on a regional basis, thereby contributing to the enhancement of Mongolia’s healthcare standards. In Cambodia, Sysmex is also supporting quality improvement in clinical testing through similar activities related to EQA in the field of hematology.
In China, its hematology reference counter has been employed as a National Standard* for Blood Cell Count since 2002, and the registration inspections and external quality assessments for all blood cell counters in China have been conducted using the reference counter provided by Sysmex as a standard. In addition, Sysmex has been providing continuous support, such as technology transfer and exchange for hematology and reference measurement procedures, while also assisting in the creation of national clinical laboratory guidelines. Since 2019, it has leased the latest standard blood cell counters, contributing to improving the accuracy and standardization of hematology tests in China.
- Analyzer with which to assign the values for the national standard of hematology (number of red blood cells and white blood cells)
Stakeholder’s Voice
“I want to ensure equal access to medical care everywhere in the world.”

In April 2025, Sysmex group’s new manufacturing base in India has begun full-scale operations, producing both diagnostic reagents and instruments.
A Sysmex employee involved in transferring instrument production technology from Japan to the new manufacturing base in India shares his passion and commitment. He is dedicated to improving global healthcare standards by promoting the high-quality manufacturing that Made in Japan represents.
Click here for details:
Delivering High-Quality Made in Japan Products to the World: Production Transfer Project to India
Public-Private Partnership Project with JICA
Sysmex Corporation conducted the Project for the Dissemination of Automated Urinalysis Diagnosis Technology between 2018 and 2022 as part of the Japan International Cooperation Agency (JICA) Collaboration Program with the Private Sector for Disseminating Japanese Technology for the Social and Economic Development of Developing Countries. We installed a fully automated urinalysis testing system in Ghana’s national Komfo Anokye Teaching Hospital (KATH), and we organized seminars and symposiums that have been attended by 860 local healthcare professionals. This project was recognized as an effort toward the attainment of the Sustainable Development Goals (SDGs), and Sysmex was certified as a “JICA-SDGs Partner.”* We will remain committed to educating local healthcare professionals on the clinical value and effectiveness of automated urinalysis testing technology in our efforts toward high quality clinical testing in Ghana and other developing countries.
- From August 2020 to February 2022
-

Presentation of JICA’s collaboration program -

Instruments installed at KATH
Public-Private Partnership with Japanese Embassies Abroad

In 2024, using a counterpart fund* accumulated under a grant aid (food assistance) framework for Niger funded through Japan’s Official Development Assistance (ODA), Sysmex installed malaria diagnostic instruments at Issaka Gazobi Maternity Hospital in Niamey and a regional hospital center in Tahoua. In conjunction with the installation, Sysmex provided training for local healthcare professionals on malaria and anemia management for malaria patients. Through these efforts, Sysmex has contributed to improve malaria and anemia countermeasures and the provision of appropriate treatment opportunities in the region.
- Counterpart funds refer to funds accumulated by the recipient country’s government through the local sale of goods and services procured under Japan’s grant aid program. These funds are used for development projects in areas such as education, healthcare, and infrastructure aimed at promoting the recipient country’s socio-economic growth, following consultations between the recipient government and the Government of Japan. The funds are managed by the recipient government, with reporting and audits conducted to ensure transparency.
Acceptance of JICA Trainees
Since 1994, Sysmex Corporation has worked with JICA in providing training to JICA trainees, in areas such as maintenance and management of analyzers and hospital management, aiming to improve healthcare workers’ knowledge and skills. The number of trainees who visited Sysmex has exceeded 1,150.
Partnership
At present, establishing medical infrastructures in response to issues at each stage of economic development is a major task for developing countries, but their healthcare markets are expected to see growth in the future. As accurate test results are a starting point for proper healthcare, Sysmex has been building relationships with the health ministry and medical institutions in each country and region, as well as creating systems for promoting high-quality testing and establishing testing environments. It has also been making efforts to create new value by utilizing international cooperation and public-private partnership frameworks and collaborating with other companies.
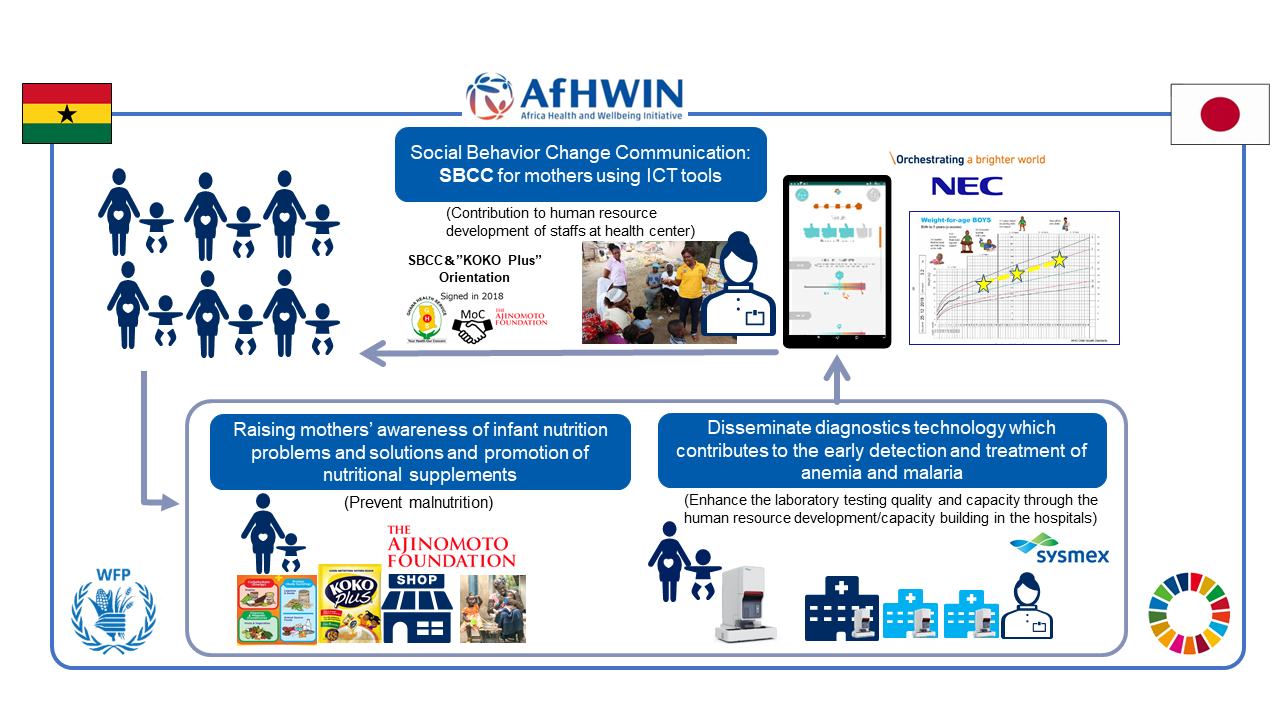
Cross-Industry Collaborative Co-creation Project: Contributing to Universal Nutrition Health Coverage
In Ghana, serious healthcare problems include malnutrition - the greatest risk factor for death and disability - and malaria, which is the leading cause of death.1 Malnutrition inhibits growth, delays the development of the body and brain in fetuses and infants, and causes anemia, increasing the severity risk for malaria. In addition, since the health of children under the age of five years and pregnant women is particularly impacted by malaria2, an integrated approach for nutrition, anemia, and malaria is required.
From 2022, Sysmex conducted a co-creation project for improving the health and nutrition of mothers and children in Ghana in collaboration with the Ajinomoto Foundation and NEC Corporation. This project further developed the Ajinomoto Foundation’s activities in collaboration with the Ghana Health Service, such as promoting behavioral changes among mothers and recommending nutritional supplements. By combining high-quality testing with ICT originating in Japan, the project has established a system that contributes to improving the health and nutrition of mothers and children. Sysmex contributed to strengthening the functions of regional hospitals by installing malaria diagnostic instruments at medical institutions and conducting training and awareness-raising activities for medical professionals. In addition, using data obtained from the malaria diagnostic instruments, we conducted joint research with the Ghana Health Service on the prevalence of anemia and malaria among pregnant women, mothers and their children, schoolchildren, and others in the project areas.
- 1 The Institute for Health Metrics and Evaluation (IHME)
https://www.healthdata.org/ghana - 2 Children under five years old are particularly vulnerable to malaria and malnutrition. Malnourished children may develop more severe cases of malaria. Additionally, malaria increases the risk of poor outcomes for mothers and newborns, such as anemia and death in pregnant women, miscarriages, stillbirths, low-birthweight infants, and newborn and infant death.
Nutrition and Malaria: Integrated approach for effective case management
Initiatives of the Business Leader’s Coalition for Global Health
Hisashi Ietsugu, Chairperson and Group CEO of Sysmex Corporation, participates in the Business Leader’s Coalition for Global Health, a group of volunteers consisting of Japanese business leaders which aims to contribute to the global health* area. In August 2022, 11 companies participating in the coalition announced “Global Health Actions” at an official side event of the 8th Tokyo International Conference on African Development (TICAD 8), with special guest Mr. Bill Gates. Sysmex made a presentation titled “Fighting malaria with diagnostics” and expressed its intention to aim for a malaria-free world. In March 2023, the Company also took the stage at the 2nd Global Health Academy to convey the significance of public-private-academic partnerships in the global health field.
In May 2024, this coalition requested the Foreign Minister at the time to position global health as a strategic area of diplomatic policy through collaboration with Global South countries. It also called for increased funding for international organizations such as Gavi, the alliance aiming to improve the global vaccine gap, as well as the resulting promotion of procurement expansion for Japanese companies’ products and services.
- Support and business development in healthcare globally, particularly in public health and measures against infectious diseases.

Exhibited at the Ninth Tokyo International Conference on African Development (TICAD9)

Sysmex exhibited at the Japan Fair, a side event of the Ninth Tokyo International Conference on African Development (TICAD9), held in Yokohama from August 20 to 22, 2025. Sysmex operates in approximately 50 of the 54 African countries and introduced its approaches to address Africa’s healthcare challenges, including disease-specific initiatives targeting anemia, infectious diseases such as malaria and HIV, and non-communicable diseases such as cancer, as well as efforts in human resource development and its service and support structure.
