Business Introduction


Diagnostics Business
Since its founding, Sysmex has focused on in vitro diagnostics (IVD) that involve the collection and examination of blood, urine, and other samples, as its core business.
IVD is utilized in various ways, including medical checkups to help prevent disease, diseases diagnosis, determination of treatment methods, measurement of drug administration results, prediction of aggravation, and post-treatment monitoring.
Accurate test results are essential because it allows medical professionals to assess a patient’s state of health accurately and promptly, and to determine optimal treatment methods.
In the IVD business domain, Sysmex provides high-quality products and services to medical institutions, clinical laboratories, animal hospitals, and research institutes.

Hematology
Hematology is a major test routinely included in blood tests. It analyzes the number, type, and size of red, white, and other blood cells. And it helps identify the possibility of diseases, such as anemia and leukemia, and can be employed to determine whether more detailed tests are required.
In the field of hematology as its main business, Sysmex has achieved the No. 1 share in the global market, supplying a wide range of products that can be used in any size of facilities.
○Related information
Hemostasis
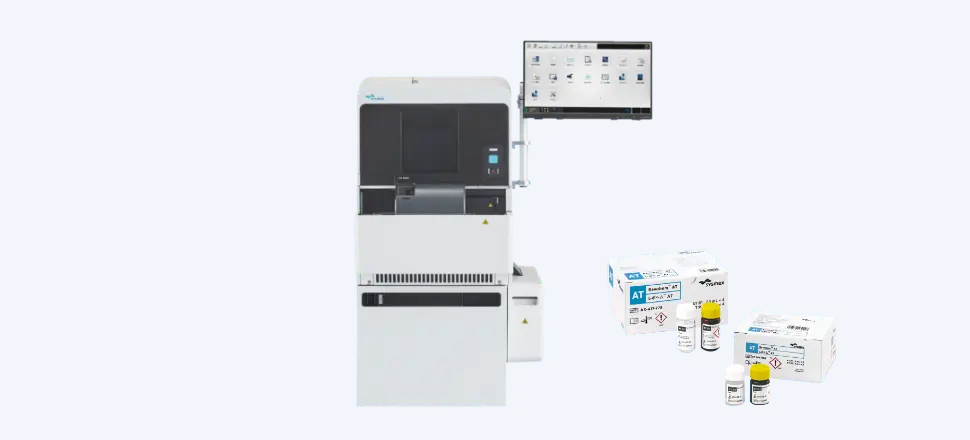
Hemostasis, a type of testing that helps determine the ability of the blood to coagulate and dissolve clots, is used when diagnosing and treating hemorrhagic diseases, such as hemophilia, and thrombotic diseases, such as myocardial and cerebral infarction.
In addition to our global OEM contract with Siemens Healthineers, we are collaborating with our group company, HYPHEN BioMed, to develop products with high clinical value, achieving the No. 1 share in the global market.
○Related information

Urinalysis
Testing to determine the presence of sugar, proteins or blood in the urine.
Urinalysis can be divided into urine chemistry, performed as the primary test, and urine sediment testing, performed as the secondary test to more closely analyze samples found to be abnormal in urine chemistry. This is used to diagnose and treat renal and urinary tract disorders.
In 1995, we developed and commercialized the world’s first automated urine particle analyzer using the flow cytometry method, and we have made a significant contribution to streamlining and standardizing urine testing. We are also adding to our portfolio of urine chemistry products by making use of alliances as we work to expand our lineup in response to diverse urinalysis needs, achieving the No. 1 share in the global market.
○Related information

Immunochemistry
Immunochemistry analyzes proteins in the blood to check for infectious diseases, such as viral hepatitis, AIDS, and COVID-19, tumor markers that indicate cancer development, hormones, and allergies.
Sysmex’s products can measure minute amounts of blood components quickly and sensitively, resulting in shorter waiting times for test results. Furthermore, we are developing new test products, such as reagents for examining the accumulation of proteins responsible for Alzheimer's disease with a small amount of blood.
○Related information

Cancer Gene Testing
Through the detection and analysis of cancer genes, this testing contributes to decisions on treatment protocols and drug administration. Using our proprietary technology, the OSNA method, we provide a system that automatically and easily detects information to assist in diagnosing lymph node metastasis. We have developed a system for use in cancer gene profiling in collaboration with the National Cancer Center Japan. The system’s targets for analysis are solid tumors. By obtaining a comprehensive cancer genomic profile of tumor tissue, the system analyzes abnormalities in cancer-specific genes in patients to provide information that is useful in determining treatment methods, including diagnoses and the selection of anti-cancer drugs. In 2019, this became the first such system to be covered under Japanese health insurance to be used in clinical settings.
Medical Robotics Business
In recent years, it has become common to perform minimally invasive laparoscopic surgery to reduce the physical burden on patients. However, this surgery requires a high degree of technical skill, and surgical support robots that complement these skills are attracting attention from medical workers.
Currently, insurance coverage of surgical procedures using these robots has been extended to include urology, gastroenterology, and gynecology, and the number of surgeries performed using surgical support robots is gradually increasing.
In addition, from the viewpoint of improving access to medical care, such robots are being considered for use with remote medicine. It may not be long before surgeries can be performed with doctors and patients in different locations.
Under these circumstances, Medicaroid Corporation, a joint venture between Sysmex and Kawasaki Heavy Industries, Ltd., developed the Japan-made robotic-assisted surgery system. As the global general distributor for this product, Sysmex began its launch in Japan in 2020.
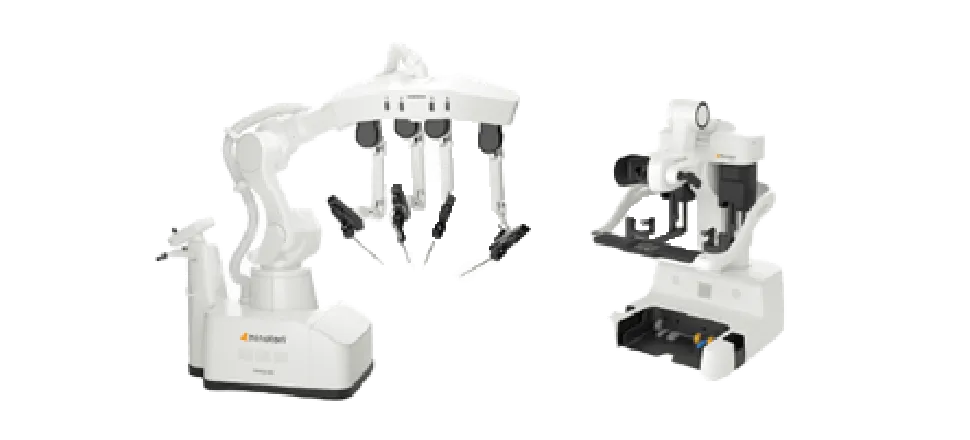
Robotic-Assisted Surgery System
Compact enough to fit in standard Japanese operating rooms, this system is equipped with user-friendly robot arms and a high-definition 3D videoscope.
Furthermore, the system has been designed to be network compatible, to support more accurate treatment by medical workers.
○Related information


Sysmex’s Research & Development
Learn about Sysmex’s research and development activities
for value added testing and diagnostic technologies.



