Stories
Tackling the Universal Threat of Antimicrobial Resistance (AMR) with New Testing Technology
- Sysmex Group Working Together in Efforts to Meet This Social Challenge -
Antimicrobial resistance (AMR) has been designated as a social challenge for the whole world to tackle. As a company involved in healthcare, Sysmex is working to establish testing technologies and product development to address this issue. In addition to explaining the importance of initiatives in AMR, we will consider AMR countermeasures in the Sysmex Group through a conversation between people working in this business area.

Antimicrobial Resistance (AMR) Spreading with Inappropriate Use of Antimicrobials
People are very familiar with antimicrobials that are used for the treatment of infections. The three major points in the proper use of antimicrobials are the following, and there are various issues to be addressed.
The first is prescribing antimicrobials properly. An effective way of achieving this is to determine the type of bacteria infecting the patient and which antimicrobial will have the best efficacy against it. However, it is currently difficult to prescribe antimicrobials based on testing results at the first consultation, because it can take several days from testing to obtaining the results. The second is not using antimicrobials unnecessarily. They may be used unnecessarily in some cases when the patient is not actually infected, because proper testing cannot be provided in a timely manner. The third is appropriate use by patients. There have been cases when, based on their own judgment, patients stop taking prescribed antimicrobials before the end of the course and use the remaining medication on another occasion.
The improper use of antimicrobials is one of the main causes of AMR. If an antimicrobial is used improperly, it will not be completely eradicated and may survive and become resistant to antimicrobials. The proliferation of drug-resistant bacteria will make antimicrobials much less effective, making it difficult to treat infections that would otherwise be mild and easy to recover from, and they may become severe or even be fatal.
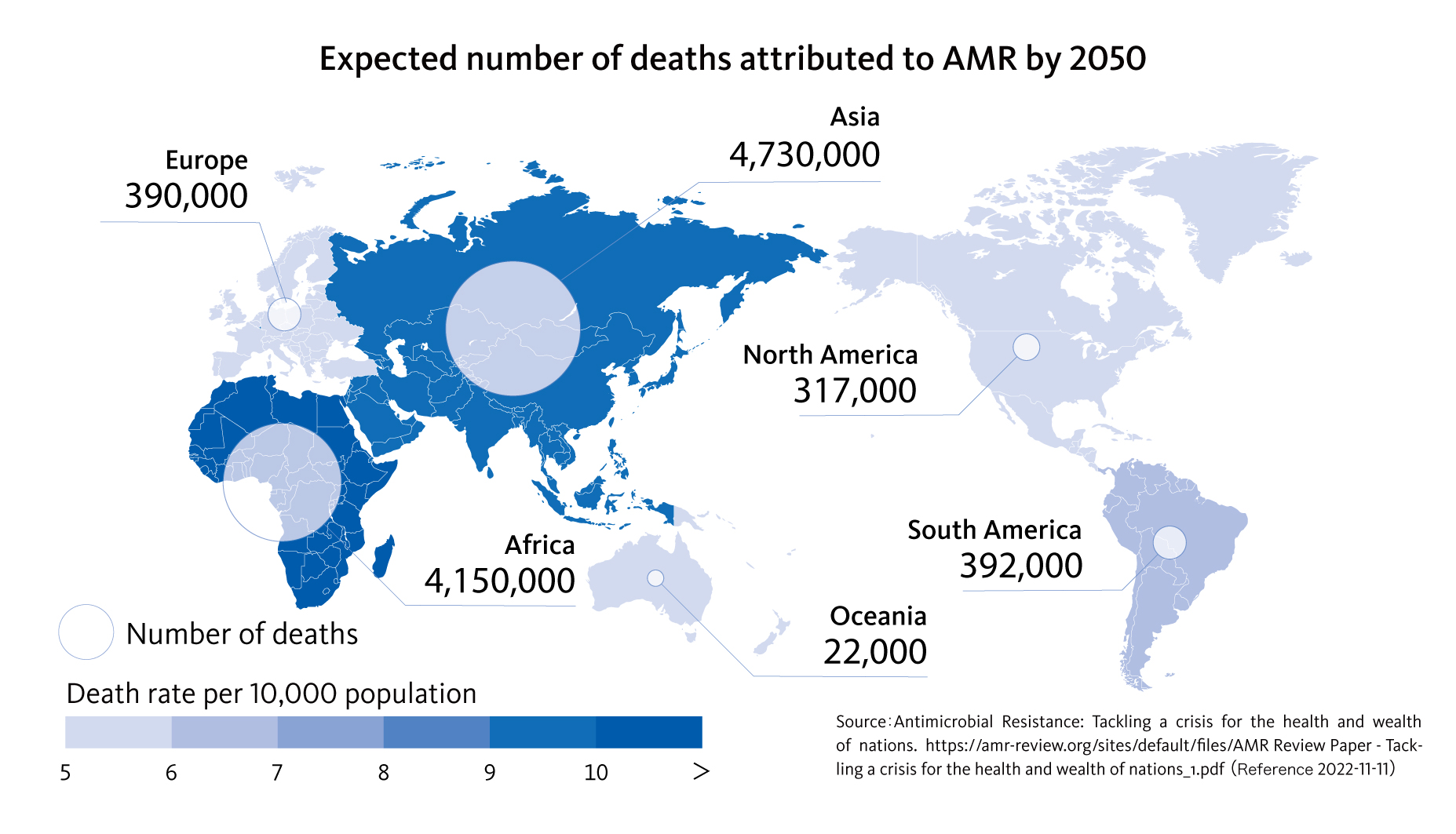
It has been estimated that 10 million people will die annually due to AMR in 2050.1 This exceeds the number of deaths due to cancer, and the WHO and various other organizations have designated AMR as a social challenge that should be addressed on a global level.
1 Source: Action Plan. https://amr.ncgm.go.jp/medics/2-4.html (Reference 2022-11-11)
The first is prescribing antimicrobials properly. An effective way of achieving this is to determine the type of bacteria infecting the patient and which antimicrobial will have the best efficacy against it. However, it is currently difficult to prescribe antimicrobials based on testing results at the first consultation, because it can take several days from testing to obtaining the results. The second is not using antimicrobials unnecessarily. They may be used unnecessarily in some cases when the patient is not actually infected, because proper testing cannot be provided in a timely manner. The third is appropriate use by patients. There have been cases when, based on their own judgment, patients stop taking prescribed antimicrobials before the end of the course and use the remaining medication on another occasion.
The improper use of antimicrobials is one of the main causes of AMR. If an antimicrobial is used improperly, it will not be completely eradicated and may survive and become resistant to antimicrobials. The proliferation of drug-resistant bacteria will make antimicrobials much less effective, making it difficult to treat infections that would otherwise be mild and easy to recover from, and they may become severe or even be fatal.
It has been estimated that 10 million people will die annually due to AMR in 2050.1 This exceeds the number of deaths due to cancer, and the WHO and various other organizations have designated AMR as a social challenge that should be addressed on a global level.
1 Source: Action Plan. https://amr.ncgm.go.jp/medics/2-4.html (Reference 2022-11-11)
Aiming to Support Medical Care Through Rapid Testing
To combat AMR, Sysmex is focusing on realizing rapid testing that will aid decisions on proper medication. Through urinalysis instruments that detect bacteria, white blood cells, and other substances in urine, Sysmex provides support for the diagnosis of urinary tract infections, and this contributes to the provision of proper treatment by reducing the unnecessary use of antimicrobials.
In addition, as a new initiative, together with a Group company, Sysmex is working on the establishment of testing technology for rapidly determining which antimicrobials will be effective (Antimicrobial Susceptibility Testing, AST), as well as product development based on it. The company is Astrego,2 which was established in Uppsala, Sweden. This company has proprietary technology capable of rapidly performing antimicrobial drug susceptibility testing, which is used to examine the effectiveness of antimicrobials against bacteria detected in urine samples. Since its establishment in 2017, this company has been going ahead with commercial development.

2 With Sysmex making Astrego a wholly-owned subsidiary in May 2022, the company’s name was changed from Astrego Diagnostics AB to Sysmex Astrego AB.
What is the partnership between Sysmex and Astrego, how far they have come, and what are their aspirations for the AMR response? From here we present a conversation between Tetsuji Umeno, Director of the Primary Care Business Development Department of HUP Business Division and Mikael Olsson, CEO of Sysmex Astrego AB.
In addition, as a new initiative, together with a Group company, Sysmex is working on the establishment of testing technology for rapidly determining which antimicrobials will be effective (Antimicrobial Susceptibility Testing, AST), as well as product development based on it. The company is Astrego,2 which was established in Uppsala, Sweden. This company has proprietary technology capable of rapidly performing antimicrobial drug susceptibility testing, which is used to examine the effectiveness of antimicrobials against bacteria detected in urine samples. Since its establishment in 2017, this company has been going ahead with commercial development.

Among its management goals, Sysmex has set “becoming a solution provider contributing to the advancement of primary care diagnostics” as a positioning goal, and has been working on leveraging diagnosis technology and IT cultivated through its in vitro diagnostics business, and providing solutions that contribute to improving access to healthcare.
In view of these circumstances, the two companies agreed to Sysmex investing in Astrego in December 2019, and since then have been working together on product development. With the objective of further strengthening synergies, Astrego became a Sysmex Group company in May 2022.
In view of these circumstances, the two companies agreed to Sysmex investing in Astrego in December 2019, and since then have been working together on product development. With the objective of further strengthening synergies, Astrego became a Sysmex Group company in May 2022.
2 With Sysmex making Astrego a wholly-owned subsidiary in May 2022, the company’s name was changed from Astrego Diagnostics AB to Sysmex Astrego AB.
What is the partnership between Sysmex and Astrego, how far they have come, and what are their aspirations for the AMR response? From here we present a conversation between Tetsuji Umeno, Director of the Primary Care Business Development Department of HUP Business Division and Mikael Olsson, CEO of Sysmex Astrego AB.

Aiming to Do Our Utmost in Meeting Unmet Medical Needs in Infectious Disease Diagnosis
| Umeno: | Sysmex’s first contact with Astrego was in 2018. I was in charge of technology exploration in the primary care business field, and came to know of Astrego through this work. I remember being greatly impressed by Astrego’s concept of meeting unmet medical needs in the diagnosis of infectious diseases. Through several conversations, I was convinced that Astrego’s technology would greatly change the field of infectious disease diagnosis. |
|
| Olsson: | Astrego saw its beginnings in research on bacteria, which began in a research laboratory at Uppsala University in Sweden. Around 10 years of trial and error led to the establishment of our microfluidic technology, which achieved rapid testing in just 30 minutes, which would normally have taken at least two days. We established our company in 2017, when we had prospects for developing our technology into a product. However, we were lacking a network for market access as well as funding, and felt that collaboration with another company would be essential for realizing a commercial product. When we were looking for a partner company, the destinies of Astrego and Sysmex became linked. |
|
| Umeno: | After deciding to invest in Astrego in 2019, we joined forces on product development. As we had achieved the initially set milestones successfully and a path to commercialization and product launch had become clear, Astrego became a member of the Sysmex Group in 2022. |
|
| Olsson: | In the last two years, we have faced many difficulties due to COVID-19, but working together with Sysmex has made the future clearer. Thanks to Sysmex’s trust in us and the strong support we have received, we have confidence in our technology. We are happy to have become a member of the Sysmex Group. |

Tackling the Major Problem of AMR, Which Can Take the Lives of Those Dear to Us
| Umeno: | We are now at the stage of further enhancing the quality of our product. Toward market launch, our focus is on educational activities to have the importance of rapid testing recognized. People know about AMR all over the world, but recognition of the testing technology established by Astrego is still low because it is new, and it is not known that it will be an effective solution for AMR. We are therefore actively engaging in communication with infectious disease specialists and other KOLs3 in Europe, the U.S. and Japan. All of them have high expectations and have shown a deep understanding of, and identified with the product concepts of, “Reducing the improper use of antimicrobials” and “Providing optimal treatment to patients,” but the real start is from now. We have to work steadily to ensure that our technology and product are adopted as a world standard to approach infectious diseases. |
|
| Olsson: | We are now faced with the major problem that 10 million people may die annually due to AMR by 2050. While prescribing antimicrobials seems to be a matter of course and everyone has used them at some time in their life, if AMR occurs due to improper use, we cannot forget that it may eventually result in someone losing their life. If rapid testing becomes widespread, it will of course be a measure against AMR. However, it should also be able to contribute in various medical situations in which antimicrobials are used, such as cancer treatment and surgical operations. As Umeno-san has said, continuous efforts are necessary. |
|
| Umeno: | Yes. It would be good if our efforts enabled a large number of people to become familiar with AMR countermeasures and access them more easily. Many people first knew about PCR tests and antigen/antibody tests through the COVID-19 pandemic. Thus, we have to increase people’s awareness of AMR to be recognized as a familiar problem. |
|
| Olsson: | The new normal is to live with COVID-19. However, AMR is a serious problem, which we know will become even more serious if things continue as they are. We have to act as soon as possible. It is only about 80 years ago that antimicrobials came into clinical use, and the way we use them is getting nearer to a state of collapse day by day. We should continually inform people of the importance of AMR countermeasures, while giving them a sense of the approaching crisis. |
3 Abbreviation for Key Opinion Leader - doctor or specialist with influence in the healthcare industry.

Aiming to Provide All People with Healthcare That Involves a Trust and Confidence Through Testing
| Umeno: | We believe that rapid testing has much to contribute, not only for patients who take antimicrobials, but also for doctors and other healthcare professionals. For example, only hospitals have microbiology laboratories to carry out the testing necessary for the proper prescription of antimicrobials; community clinics do not have them. Owing to this, the majority of doctors can only prescribe antimicrobials based on experience, not on test results. Regarding our testing technology, one doctor told me that being able to prescribe medicine to patients based on evidence provided a sense of ease. I realized that testing is directly linked to the relationship of trust between doctors and patients. Being able to conduct testing easily at any time and see the results quickly not only raises the efficiency of medical care and the patient’s QOL, but also results in a sense of ease for both healthcare professionals and patients. |
|
| Olsson: | Some things that doctors have said have made an impression on me. It seems that quite a few patients come back saying, “The antimicrobial you prescribed is not effective; please prescribe another.” If these doctors had our technology, it would provide a solution. Another doctor asked me if we could make a product that could be used in septicemia testing. The product we have developed uses a basic platform for testing. By varying the application, we can develop products that can be used for a variety of diseases. Believing in our potential, we will continue working toward a world in which less people have a negative experience due to AMR. |

Sysmex has worked on establishing new testing technologies and product development over many years, and Astrego has developed its own technology that enables rapid testing. The coming together of the two companies and their synergies has resulted in lighting the way in the area of infectious disease diagnosis. Going forward, through the development of innovative testing and diagnostic technologies and their provision in products, the Sysmex Group will continually seek solutions for AMR and other medical problems to support the healthy lives of all people.
Related Information
- What Is Antimicrobial Resistance (AMR)?
- Sysmex Astrego: Providing Antibiotic Resistance Testing (CBS News Global Thought Leader)
- Sysmex joins the fight against antimicrobial resistance (Sysmex Europe)
- Sysmex Joins the Fight Against AMR - Sysmex (sysmex-ap.com)
- Sysmex’s PA-100 AST System for Rapid Detection of Antimicrobial Susceptibility Wins One of the UK’s Biggest Science Prizes “Longitude Prize on AMR” *Updated on June 13,2024
Source: Official Website of Longitude Prize on AMR
Related Keywords
- Information contained in the stories is current as of the date of the announcement,
but may be subject to change without prior notice.
