Stories
Shedding Light on Intractable Eye Diseases with Genetic Testing
- Easing the Anxieties of Patients and Their Families Through Testing and Diagnostics -
The Current Situation and Challenges Surrounding Patients with Rare and Intractable Diseases, and the Future of Genomic Medicine
Approximately 80% of rare diseases are considered genetic disorders due to abnormalities in genes,1 and many are regarded as fundamentally difficult to treat.
However, in recent years, significant advancements in genomic analysis technology have begun to reveal the differences in phenotype for each causative gene. With the development of gene therapy technologies, the application range of gene-based treatments has greatly expanded, and it has become possible to enhance treatment efficacy.
Sysmex, dedicated to developing innovative testing and diagnostic technologies to enhance individual healthcare journeys, has leveraged its experience and know-how in implementing cancer genomic medicine in Japan to explore the potential of gene panel testing for rare and intractable diseases. One significant achievement is obtaining the first regulatory approval in Japan for gene panel testing targeting the rare retinal disease IRD.
The Challenge of Clinically Implementing Gene Panel Testing for Inherited Retinal Dystrophy (IRD)
Venturing Into Uncharted Territory with a Desire to Ease the Anxieties of Patients and Their Families

“Due to the difficulty in diagnosing these conditions, patients must often endure a ‘diagnostic odyssey,’ being sent from one medical institution to another for years. Although it doesn’t take long to diagnose IRD, the visual function prognosis and inheritance patterns, which affect the likelihood of the appearance of the genetic disorder, vary depending on the causative gene. Clinical research has shown that comprehensive examination and identification of the causative gene is crucial for appropriate diagnosis, treatment, and social care. However, at the time, there were no comprehensive genetic tests available for clinical use to identify the causative genes. Listening to doctors’ stories, I realized that patients diagnosed with IRD have many concerns, not only about their own condition but also about their children’s future. Testing can connect patients to personalized care and medical treatment, and knowing the cause of their disease allows them to understand their condition and make informed decisions about their future. In this sense, the role of testing and diagnostics is extremely significant.”
Watanabe reflects on the early days of the development:
“We understood that developing a test for a disease with limited treatment options would be challenging. However, not pursuing it because it was a rare disease was never an option. Within the company, there were various opinions about expanding into rare and intractable diseases, but we were determined to meet the expectations of the medical field and persuaded the team. We were confident that our experience in clinical implementation—from cooperation in the establishment of systems to regulatory approval and insurance coverage—would be invaluable. We felt it was our mission to ease the anxiety of patients and their families as much as possible through the power of testing and diagnostics.”
The Value of Knowing the Causative Gene: Contributing to Supportive and Holistic Care Through Testing and Diagnostics
Project leader Hayato Niiro explains, “Recently, the concept of ‘Shared Decision Making (SDM),’ where the doctor and patient collaborate to make medical decisions by sharing medical information, the best evidence, and the patient’s background and values, has been emphasized. The development of gene panel testing that comprehensively examines the genes causing IRD is precisely the kind of initiative that contributes to the SDM approach.”

Designing a System to Connect Testing to Diagnosis and Overcoming Clinical Implementation Barriers Through Industry-Academia-Government Collaboration
Regarding Sysmex’s role in clinical implementation, Watanabe says:

Continuously Support Patients’ Healthcare Journeys Through Testing and Diagnostics
With the introduction of Sysmex’s gene panel test, IRD treatment and care entered a new phase.
Niiro says, “In identifying causative genes and considering subsequent treatment and social care, a process has emerged where experts from various fields, including ophthalmologists, geneticists, and genetic counselors***, discuss the findings based on scientific evidence from test results. This embodies the concept of ‘team-based care,’ where healthcare professionals with various expertise collaborate to provide treatment and care.”
“In many disease areas, research is progressing on innovative gene therapies and drug treatments based on causative genes, which can improve prognosis. For these treatments to be established, tests that identify the relevant causative genes are necessary. Leveraging this experience, we aim to advance the evolution of treatment and care for rare and intractable diseases through testing and diagnostics.”

“Leveraging our experience in cancer genomic medicine and the field of rare and intractable diseases, we aim to deliver testing and diagnostic solutions that meet unmet medical needs as quickly and with as little burden as possible. We provide information that helps patients and their families understand and face their illnesses with confidence. This is what Sysmex, which has been pioneering new values in testing and diagnostics for over 50 years, is expected to do.”

Column | What is Inherited Retinal Dystrophy (IRD)?
IRD is a collective term for genetic, progressive diseases caused by gene abnormalities that impair the retina’s ability to sense light. Retinitis pigmentosa, a representative disease, is the second leading cause of visual impairment in Japan, following glaucoma. Symptoms and their progression vary among patients, but typically include difficulty seeing in dim light beginning at an early age, a narrowing of the visual field, and decreased vision with some cases leading to blindness.
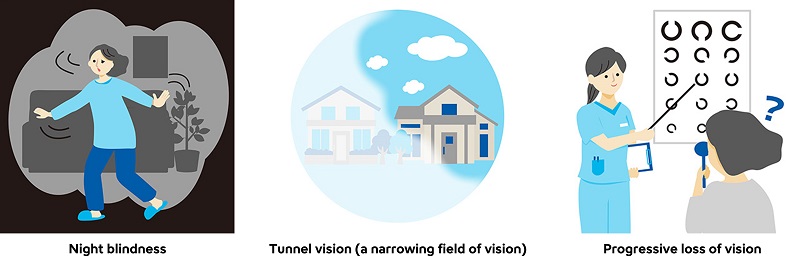
Main Symptoms of IRD Patients
Nearly 300 genes have been reported to play a crucial role and lead to IRD. Although IRD is a genetic disease, not all patients pass it on to their children. For example, in retinitis pigmentosa, clear inheritance is observed in only about half of the cases.2 Symptoms and progression can vary significantly depending on the type and location of the gene variant, and even with the same causative gene, the diagnosed disease name may differ.
Terminology
| * | Gene panel testing examines multiple genes simultaneously to identify genetic mutations associated with diseases. Genomic medicine involves comprehensively examining an individual’s genomic information through gene panel testing and using the results to diagnose and treat diseases more efficiently and effectively. |
| ** | Low vision care: A comprehensive term for support provided to individuals with visual impairments that affect their daily lives. This includes medical care, educational, vocational, social, welfare, and psychological support. |
| *** | Genetic counseling: According to the Japanese Association of Medical Sciences, this process helps individuals understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease. It includes counseling to promote informed choices and adaptation to risks and conditions. |
References
| 1. |
International Federation of Pharmaceutical Manufacturers & Associations: IFPMA, “Rare Diseases: shaping a future with no-one left behind ”
https://www.jpma.or.jp/english/globalhealth/status_effort/2018/eki4g6000000495y-att/2018_e_02.pdf (viewed October 1, 2024) |
| 2. |
MHLW Grants System, Policy Research Project on Intractable Diseases, Research Group on Retinal, Choroidal, and Optic Nerve Atrophy. Working Group for the Development of Clinical Practice Guidelines for Retinitis Pigmentosa. Clinical Practice Guidelines for Retinitis Pigmentosa. Journal of the Japanese Ophthalmological Society. 2016;120(12):846-861. *Japanese Only
https://www.nichigan.or.jp/Portals/0/resources/member/guideline/retinitis_pigmentosa.pdf (viewed October 1, 2024)
|
Related Keywords
- Information contained in the stories is current as of the date of the announcement,
but may be subject to change without prior notice.
