Sysmex’s Growth Strategies


In the Long-term Corporate Strategy 2033, Sysmex states its Long-term Vision of “Together for a better healthcare Journey.”
In addition to creating innovation in in-vitro diagnostics to ensure a better healthcare journey for each individual over its life, we will offer new value by meeting the challenges in prevention, pre-symptomatic, and treatment and aim to be a company with ¥1 trillion or more in net sales in fiscal 2033.
Toward these goals, we have set three growth strategies.
Growth Strategies
Business Expansion in Emerging Countries
Exporting products to over 190 countries and regions, Sysmex has business in markets ranging from those in developed countries to those in developing countries.
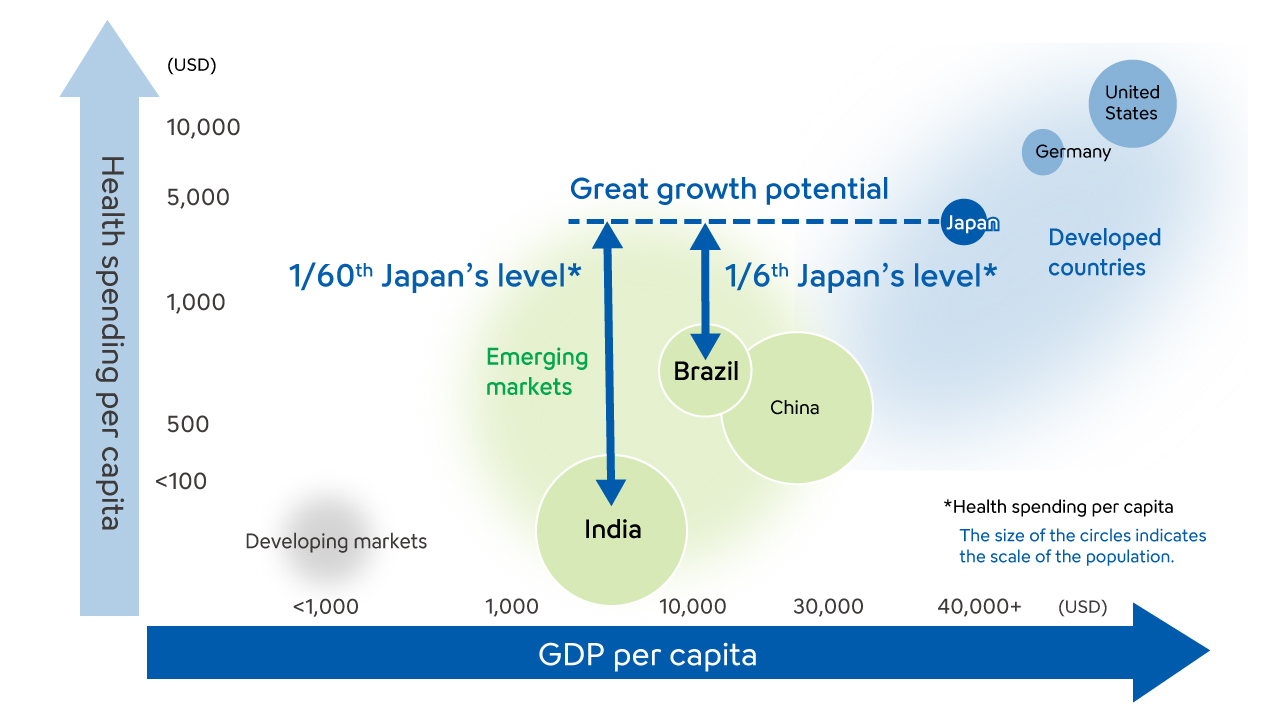
Emerging markets, which account for approximately 35% of Sysmex sales, are expected to continue seeing growth in the future due to improvement of economic standards and quality of healthcare.
For instance, in India, which boasts the largest population in the world, national healthcare expenditure per person is around 1/60th Japan’s level, while that in Brazil, the most populous country in South America, is around 1/6th Japan’s level, so there is huge market growth potential.
To seize opportunities for business growth, Sysmex will aggressively invest in these markets that are expected to experience significant growth in healthcare demand, including India, Central and South America, and the Middle East and Africa.
The Potential Healthcare Market
Total Sales in India, Brazil, the Middle East, and Africa
India


Sysmex began its business in India with the establishment of a joint venture (current Sysmex India Pvt. Ltd.) in 1998. In 2007, a reagent production factory was established, and the joint venture became a wholly-owned subsidiary in 2008. Since 2019, we have been promoting significant business expansion and the expansion of our sales and service networks by gradually expanding direct sales, service and support in the mainstay area of hematology.
In India, where high economic growth is expected to continue, we have established a new manufacturing base in Gujarat State in consideration of responding further increases in testing demand. It stands as the Sysmex Group’s largest manufacturing base and equipped with production capabilities for both diagnostic reagents and instruments.
Under the “Make in India” policy to foster economic growth, job creation, infrastructure, India is implementing this policy into public procurement to favor domestically manufactured products. Sysmex has achieved mass production of its instrument at the new manufacturing base in India, meeting the participation requirements for public procurement (local production and incorporation of local materials). These enhancements strengthen the stable supply of products to the Indian market, improve customer satisfaction, and aim to accelerate business expansion.
Brazil

In 1998, Sysmex established our first local subsidiary in Central and South America (Sysmex do Brasil Industria e Comercio Ltda.) in Brazil. In 2000, a reagent factory was also established. We have expanded our business in Central and South America region centered on Brazil. Furthermore, since 2014, Sysmex has established local subsidiaries in Mexico, Colombia, Chile, and Argentina, expanding its business in these regions.
In order to further enhance and strengthen customer support in the Central and South American market, which is expected to continue growing in the future, we have opened a new support base in Brazil in November 2023. Training for the customers in Central and South America, which was previously delivered online from our support base in North America (Chicago) can now be delivered locally, enabling us to respond to local needs and provide higher level support in the market, including face-to-face practical training.
Reinforcement of Existing Business
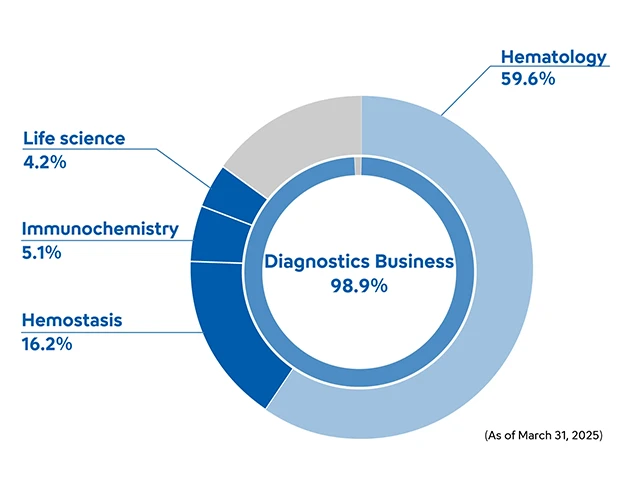
Sysmex’s business centers on in vitro diagnostics, which involves testing blood, urine and saliva samples taken from the body.
Going forward, following hematology field which has the top global share and is the main pillar of our earnings, we will focus on reinforcing the three other fields of hemostasis tests, immunochemistry tests and life science for which further sales growth and increased profitability are expected.
In addition, we tailor our business to local characteristics through direct communication with customers to strengthen and expand direct sales and service system for proposing solutions to various issues at the site of medical practice, as well as alliances with global companies.
Hemostasis testing
Start of direct sales in the hemostasis testing domain in Europe and Americas
Hemostasis tests are used to determine the clotting and dissolution capacities of blood and are used for diagnosis of myocardial infarction and checking clotting capacity prior to surgery.
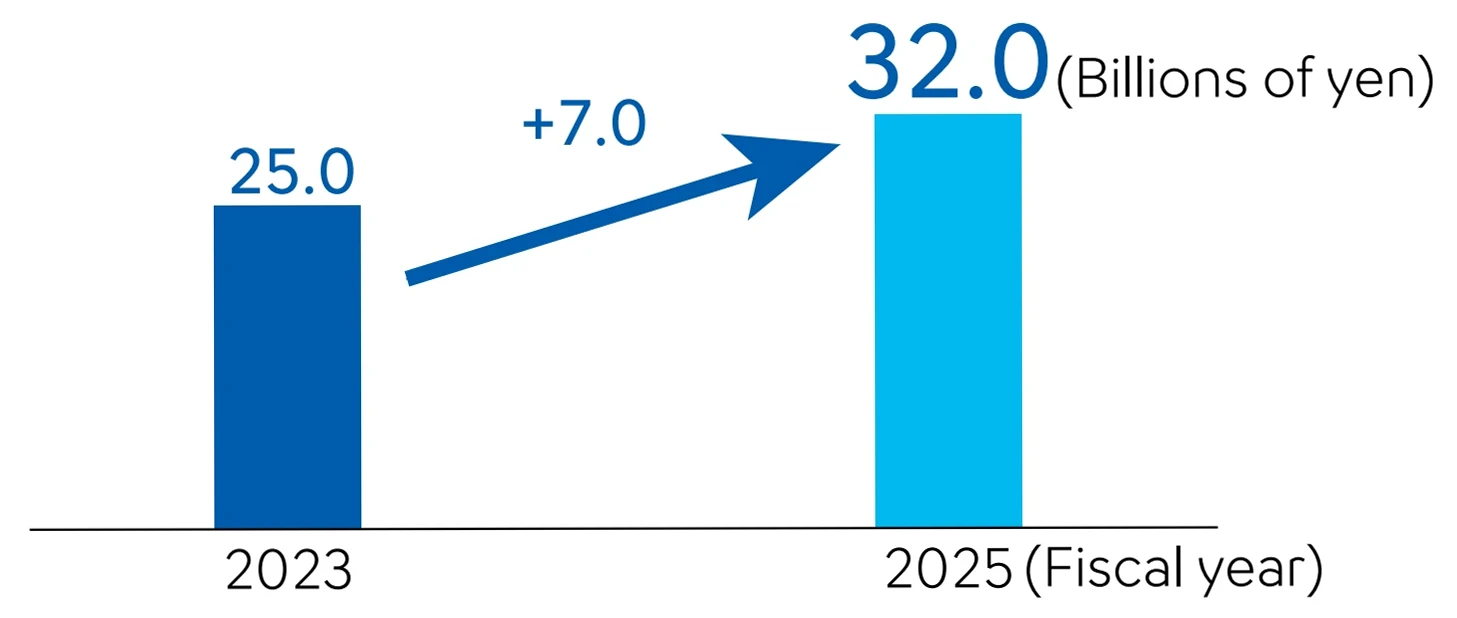
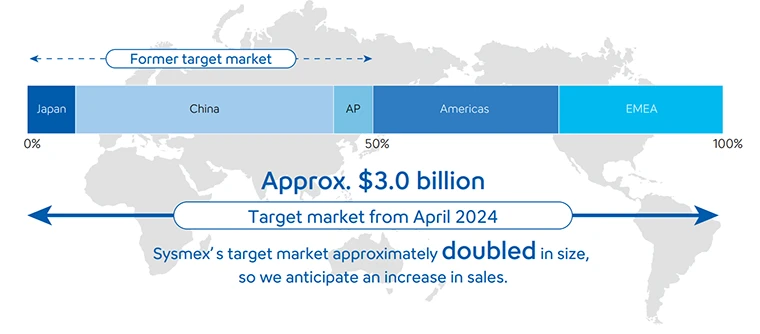
Since entering into a global alliance agreement with Siemens Healthineers in 1995, the two companies have together acquired the top global share of the market in the hemostasis field Under the previous alliance, the two companies had been responsible for separate territories in selling the products. However, in the renewed agreement of 2023, this arrangement was changed to sell products and services, globally and individually, under their respective brands. Owing to this, in April 2024, Sysmex’s sales territories expanded to include the Europe and the United States. In addition to increasing sales, we also aim to improve profitability by selling not only instruments but also highly profitable reagents in new regions.
Regional Market Size of Hemostasis Testing Field (2022)
Leadership Visions on Sysmex Hemostasis Expansion
Immunochemistry
Driving global development with the strength of unique testing parameters
Immunochemistry tests, which determine the status of proteins, viruses and hormones in blood, are the largest field in the diagnostics market. Sysmex has focused on developing unique reagent parameters that leverage the features of its products in the Immunochemistry field. As an example of this, we have developed a reagent for determining the accumulation status of a protein considered to be the cause of Alzheimer’s disease from small amounts of blood. Reducing the burden on patients compared with conventional methods such as PET scans or cerebrospinal fluid tests, it is expected to expand the opportunities for patients to receive appropriate testing.
Through the sales of this Alzheimer's disease testing reagent in the United States and Europe, and the expansion of our immunochemistry testing parameters at an early date by leveraging the effective use of alliances, we will expand our immunochemistry testing business, which has been centered in Asia, into full-scale global operations. Going forward, we will actively promote initiatives to promote acquiring regulatory approval and insurance coverage in each region.

- Rapid analysis- 17 minutes
- High sensitivity
- Low sample volume
- Unique reagent lineup
Life science
Improve profitability through products with particular strengths
Sysmex entered the life science market centered on cancer gene testing in 2000. We have developed technologies such as a cancer lymph node metastasis testing system using the OSNA™ method1 and has utilized them into products.
Going forward, we will further improve profitability by globally expanding the above systems which have already generated stable earnings in some regions and PCR testing products.
In addition to revising our product portfolio which includes laboratory assay business where we receive samples from medical institutions and return the results, we will expand our life science business through open innovation.
Open Innovation to accelerate R&D
The number of medical institutions using cancer genome medicine is limited due to the high cost and complicated testing procedures.
In order to resolve this issue, Sysmex entered into an agreement with Hitachi Hi-Tech to conduct joint research on a new rapid genome testing system that would be low in cost and easy to use at the medical field. It is our aim to have genome analysis appropriate to individual diseases used by large numbers of medical institutions.

- High quality instrument design technology
- Simultaneous development of instruments and reagents
- Global sales network
- NGS2 reagent development technology
- NGS analysis technology

- High-performance instrument design technology
- Capillary electrophoresis sequencer technology and instruments
- OSNA (One-Step Nucleic Acid Amplification): A technology developed by Sysmex enabling one-step gene amplification without the need for nucleic acid extraction and purification in pre-processing.
- NGS (Next generation sequencer): Analysis device that reads the genetic information in a large number of DNA bases and their sequences in parallel at the same time.
Strengthening direct sales and service system
In Italy, where we had provided sales and service for over more than 30 years via distributors, Sysmex started direct sales and service in the fields of hematology, urinalysis, and hemostasis in April 2024. We will expand our business by increasing our share of the market by offering solutions to diverse issues through direct communication with our customers.
Interview with Managing Director of Sysmex Italy
Expansion of New Businesses
Toward the realization of its long-term vision of “Together for a better healthcare journey,” Sysmex is taking on the challenge of expanding the scope of its business beyond the mainstay of in vitro diagnostics business. We aim to strengthen and expand business areas for which medical needs are expected to increase in the future, such as medical robots, regenerative and cellular medicine and digital medicine, through collaboration with alliance partners.
Medical robots
Since medical robots enable surgery without making large incisions at the surgical site, they contribute to reducing the physical burden on the patient and their early return to a normal life. There are also high expectations on medical robots in realizing precision surgery and remote surgery.
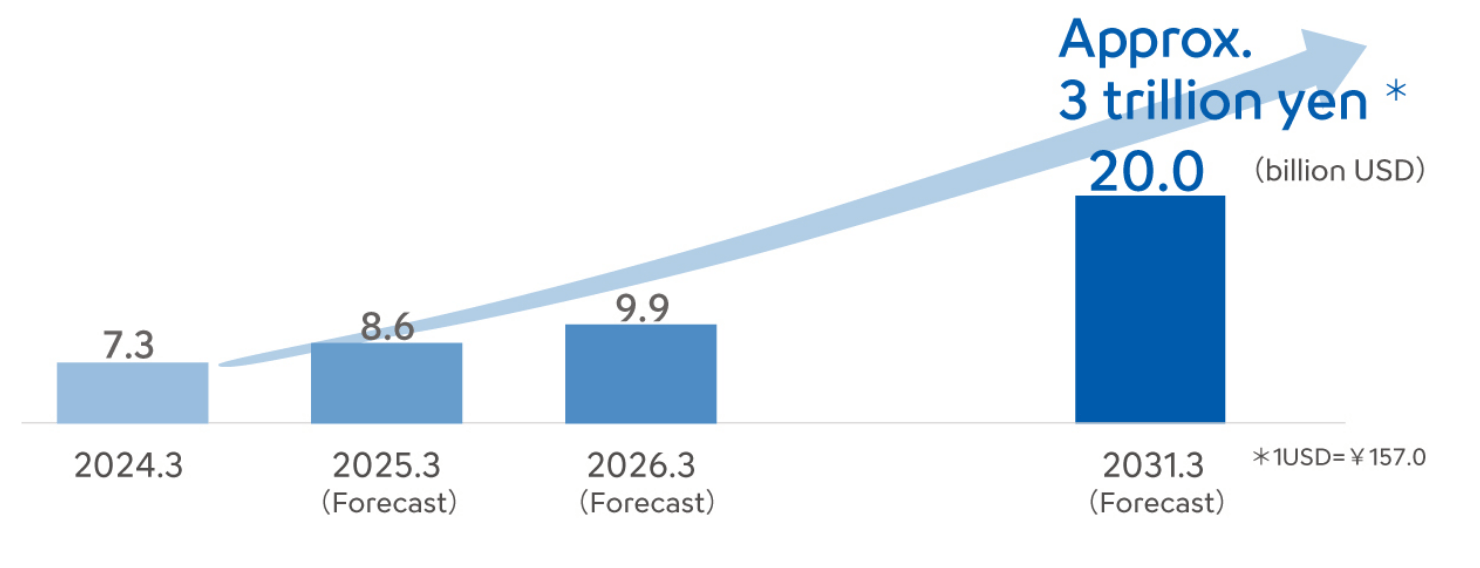
The global market is forecast to grow to roughly 3 times its current size by 2030.
Medicaroid Corporation, the joint venture between Sysmex and Kawasaki Heavy Industries Ltd., has developed a surgical support robotic system originating in Japan and has been selling it since 2020. Sysmex is the exclusive distributor for global sales of the product.
We expanded the range of applicable medical specialties, allowing the system to support over 90% of robot-assisted surgeries in Japan. Going forward, aiming to accelerate our rise in the number of units installed, we will strengthen our sales promotion activities to enlarge our installed base, focusing on nation-wide university hospitals and healthcare facilities that are currently using our products. Starting from Asia, we will also prepare for global expansion, aiming to obtain regulatory approval in Europe and the United States at an early stage.
Global market for surgical support robots
Regenerative and cellular medicine
Regenerative and cellular medicine involves adjusting the function of stem cells to convert them into cells needed to restore function in patients with dysfunctional or diseased body tissues or organs. It is attracting attention as a technology that can treat diseases that have been difficult to be cured completely. The market is expanding rapidly and expected to continue growing at a high rate in the future.
In order to provide regenerative cell medicine, to a wide range of patients, it is important to resolve issues related to cell manufacturing, such as the cumbersome manufacturing processes and unstable quality.
Sysmex aims to address these challenges by leveraging its expertise in the cell assessment technologies and digital platforms it developed in the hematology field to improve quality and automate processes. This will allow regenerative cellular medicine to reach a larger number of patients. Furthermore, by promoting the development of regenerative medicine products through open innovation, Sysmex will contribute to the societal implementation of regenerative medicine.
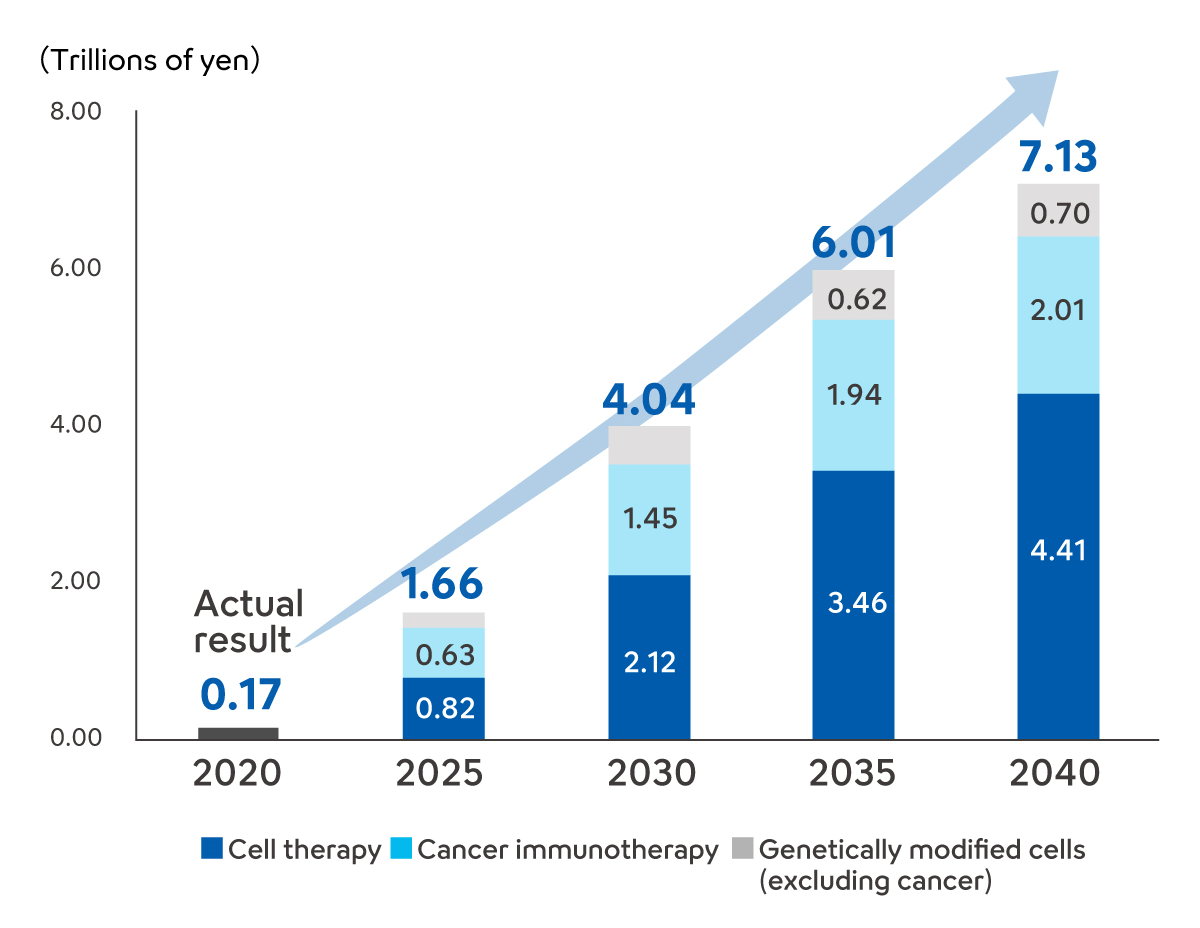
Global Market Forecast for Regenerative Cell Medicines
Digital medicine
In recent years, innovations in medical technologies have led to the provision of digital medicine. Digital transformation of medical care through the analysis and utilization of diverse medical data has the potential for new medical care that enables the management of comprehensive information in the healthcare journey of each patient and optimal support in each process.
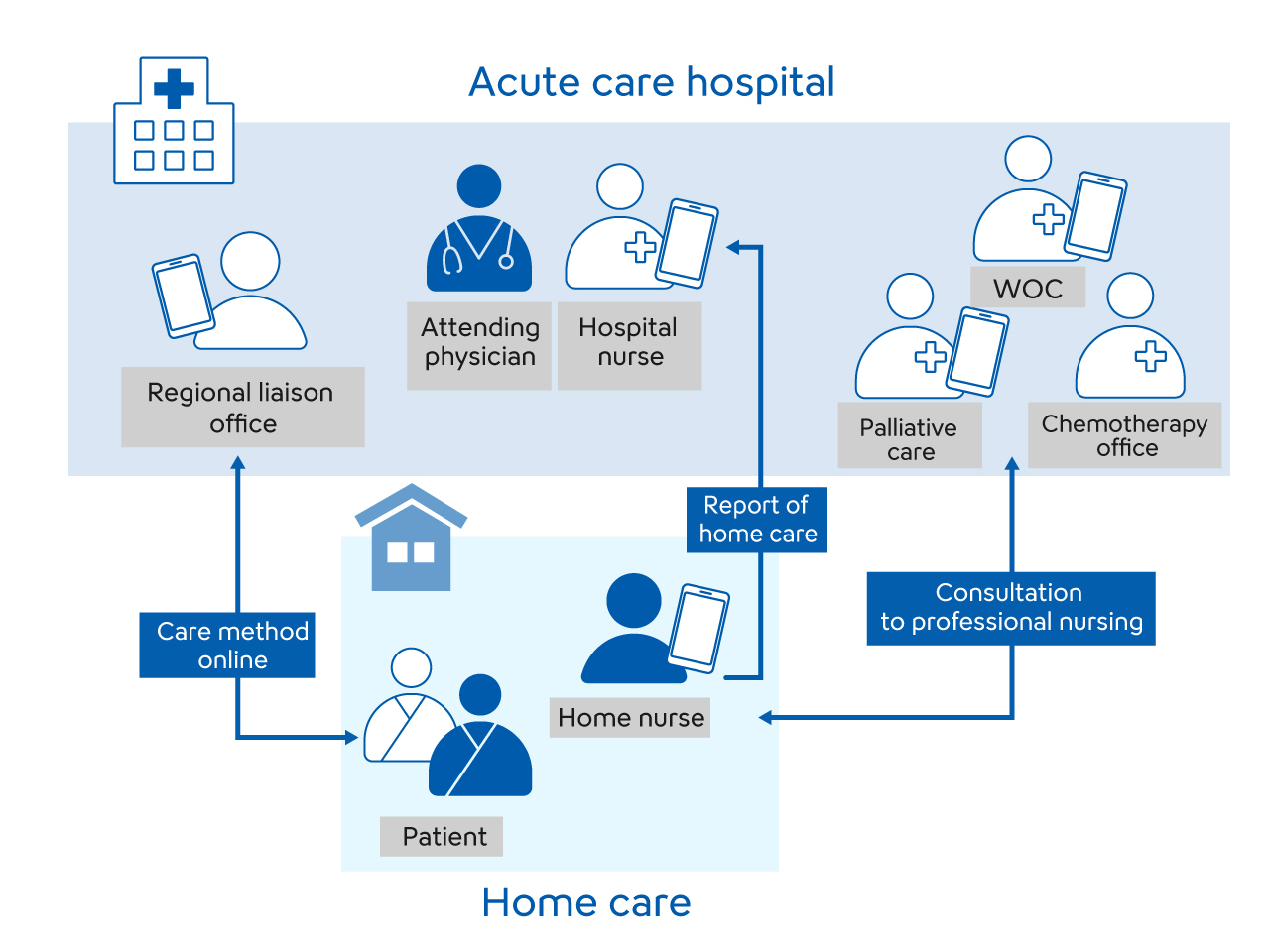
In June 2020, Sysmex and OPTiM Corp., which has strengths in AI and IoT, established a joint venture D’PULA Medical Solutions Corporation. The objective of this is accelerating the commercialization of digital medicine to support next generation medical care and diagnosis. As its first platform, the joint venture has released an app for nurse-to nurse collaboration to support more attentive home care for patients by streamlining coordination between hospitals and home care stations through digitalization.
By developing and commercializing medical applications and IT platforms applying advanced AI and IoT technologies, we will develop and provide diverse solutions to make a contribution to the long-term development of medical care for the society of the future.
Nurse-to nurse collaboration APP "Kaleido TOUCH™
touch.com/ *Japanese only