News
News
Sysmex Partec’s CyFlow™ Counter System, a System to Test for CD4+ Lymphocytes, Receives WHO Prequalification
-Aiming to Help Improve the Quality of HIV Diagnosis and Treatment in Emerging Markets and Developing Countries-
Sysmex Corporation (HQ: Kobe, Japan; Chairman and CEO: Hisashi Ietsugu) provides notice that the CyFlow™ Counter System (CyFlow™ Counter with CD4 easy count kit and CD4% easy count kit), developed and produced by its subsidiary Sysmex Partec (“Partec”), has received WHO prequalification.1 This testing system helps diagnose and monitor the immune status of people infected with HIV by checking CD4 in human blood samples. This marks the first time a Sysmex Group product has received WHO prequalification. The United Nations and other procuring agencies use WHO prequalifications as a reference when making decisions to purchase in vitro diagnostics products. Obtaining this prequalification should help broaden the reach of the CyFlow™ Counter System into emerging markets, developing countries, and other countries and regions where medical resources are limited.
Sysmex provides the CyFlow™ Counter System, a CD4 testing system developed and produced by Partec, to a wide range of customers, ranging from health centers in emerging markets and developing countries to clinics, public hospitals and laboratories of core local hospitals.
In sub-Saharan Africa, where HIV is rampant, and in other countries and regions where medical resources are limited, international funds and organizations such as the United Nations and the Global Fund work to provide assistance in procuring such items as in vitro diagnostics instruments and reagents. They typically make WHO prequalification one of their purchasing conditions.
WHO prequalification is a certification system indicating the WHO’s endorsement of quality, safety and efficacy, helping to provide peace of mind when using diagnostic instruments and medicines in countries where such resources are limited. In obtaining certification for this system, Sysmex and Partec conducted performance evaluations to ensure the system satisfied quality and safety requirements.
The CyFlow™ Counter System, which has received WHO prequalification, comprises a compact, robust desktop flow cytometer (CyFlow™ Counter) and a group of related reagents (CD4 easy count kit/CD4% easy count kit). The system measures, within three minutes, the number and percentage of CD4+ lymphocytes in the blood without the need for reference beads3, simplifying maintenance. This portable system with its high processing capability and measurement accuracy using flow cytometry technology facilitates simple, swift and stable testing. This system has obtained CE Marking (European Conformity) certification, which is a mandatory conformity marking for certain products sold within the European Economic Area.
The Company will take this WHO prequalification as an opportunity to promote the introduction of the system, including in countries where medical resources are limited. In doing so, we will help improve the quality of HIV diagnosis and treatment in emerging markets and developing countries.
Going forward, Sysmex will continue to leverage the technologies and business expertise cultivated in the field of in vitro diagnostics to provide products and services that help resolve healthcare issues and improve access to healthcare in regions around the world. In this manner, we aim to instill trust and confidence among customers around the world, including in emerging markets and developing countries.
Overview of the Obtained WHO Prequalification of In Vitro Diagnostics
| Product name: | CyFlow™ Counter System with CD4 easy count kit and CD4% easy count kit | |
| *Composed of a flow cytometer (CyFlow™ Counter), and related reagents (CD4 easy count kit/CD4% easy count kit) | ||
| Intended use: | Measure number and percentage of CD4+ lymphocytes | |
| Date of listing: | August 8, 2018 |
System Photos

CyFlow™ Counter
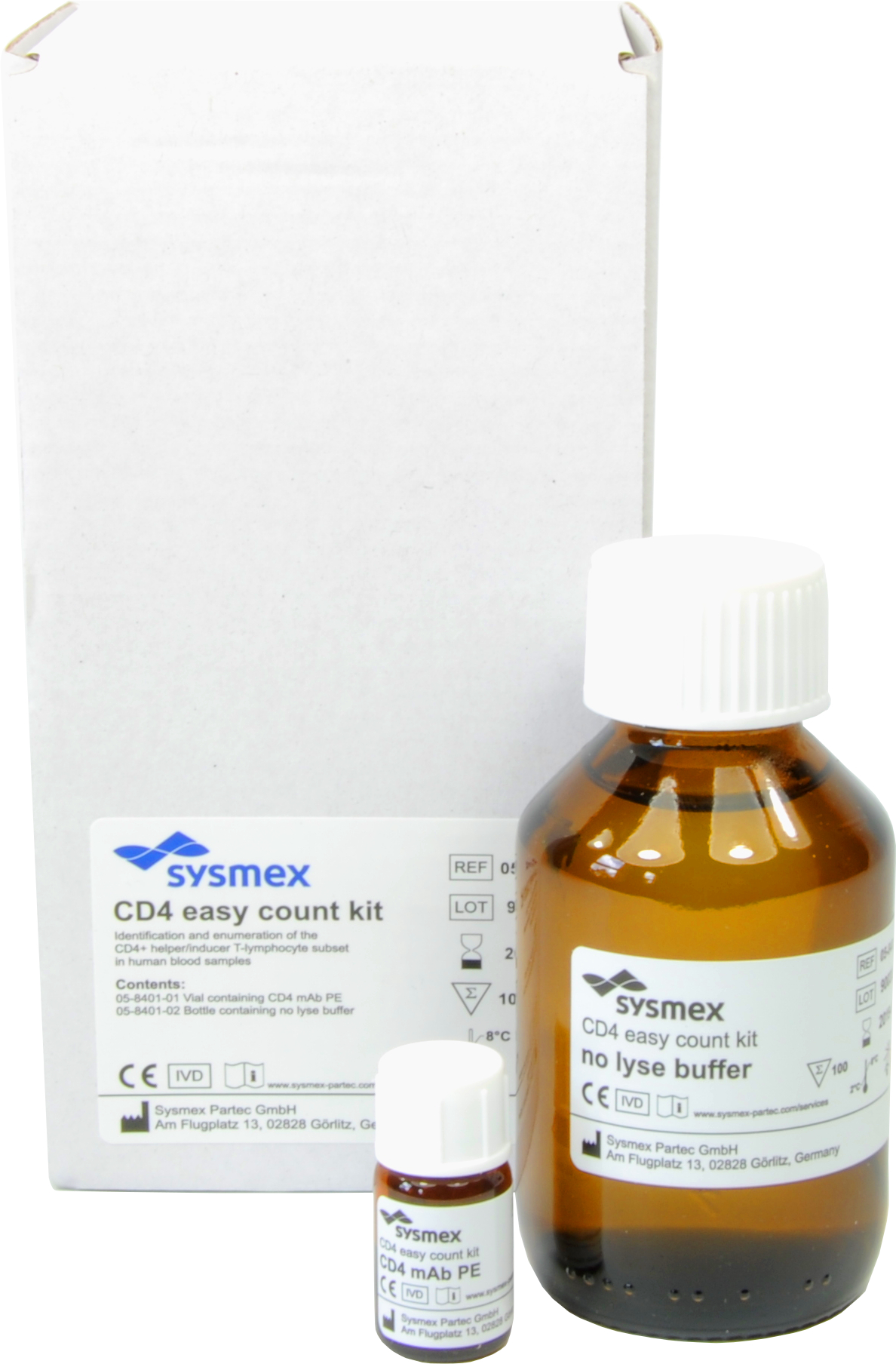
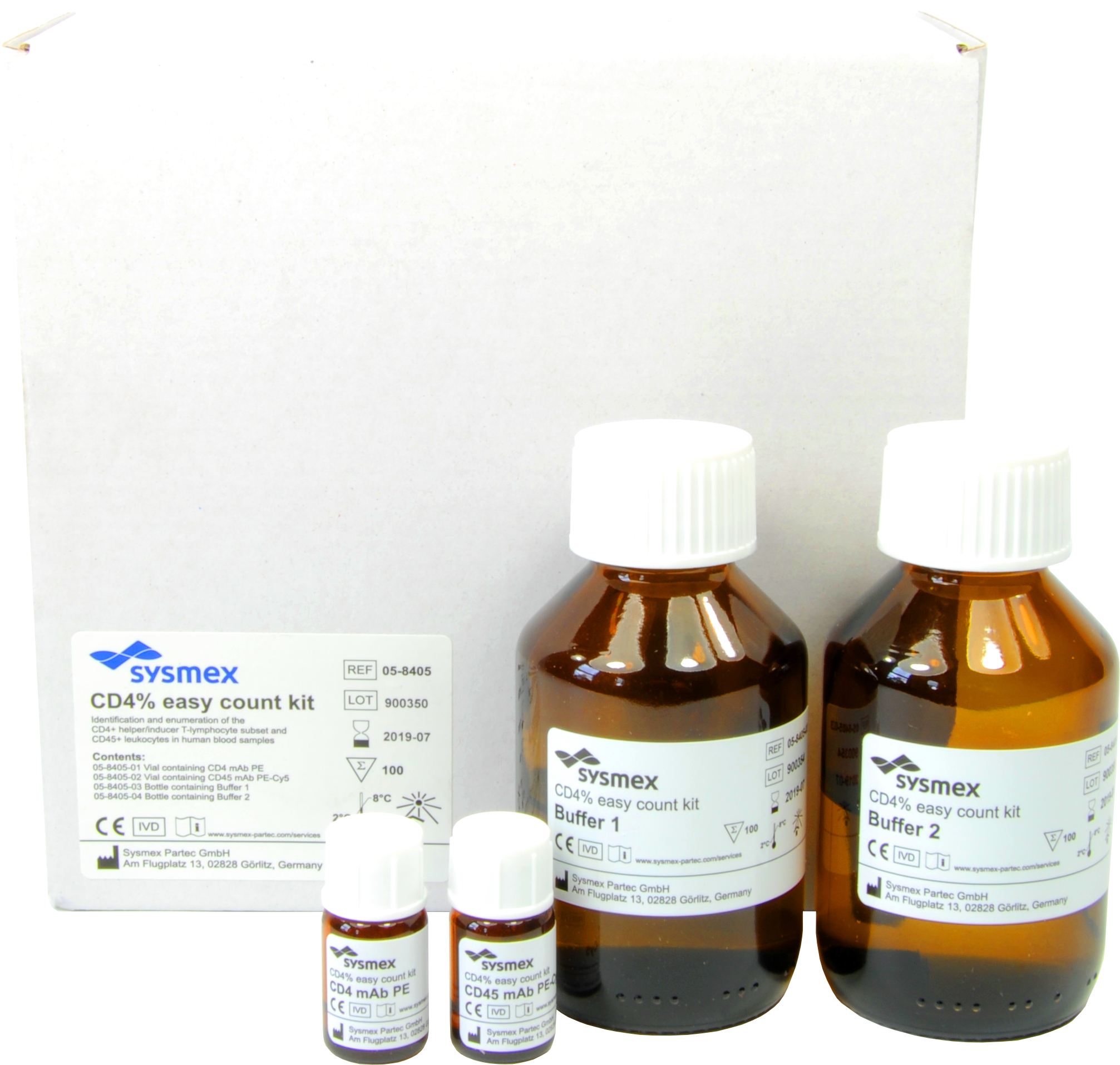
CD4 easy count kit / CD4% easy count kit
Terminology
| 1 | WHO prequalification WHO prequalification aims to ensure that diagnostics, medicines, vaccines and immunization-related equipment and devices for high burden diseases meet global standards of quality, safety and efficacy, in order to optimize the use of health resources and improve health outcomes. The system was established in 2001 in response to the HIV/AIDS pandemic. Today, it is used as a reference list for purchasing in emerging markets and developing countries. Funds such as the Global Fund give purchasing priority to products with this prequalification. |
|
| 2 | Source: Number of people (all ages) living with HIV (WHO, 2017) |
|
| 3 | Reference beads: Beads used as a reference when an accurate cell count is required, to clarify the concentration in advance |
- Information contained in the news release is current as of the date of the announcement,
but may be subject to change without prior notice.
