News
News
Sysmex Starts Joint Research with National Cancer Center Hospital to Verify Clinical Usefulness of Technology for Detecting Circulating Tumor Cells
Sysmex Corporation (HQ: Kobe, Japan; President: Hisashi Ietsugu) has started research jointly with the National Cancer Center Hospital (Location: Chuo-ku, Tokyo, Japan; President: Setsuo Hirohashi) to verify clinical usefulness of a technology developed by Sysmex for detecting living tumor cells suspended in blood (CTC detection technology).
The prognosis for a patient with metastatic cancer is very poor. Accordingly, early detection of possible metastasis is crucial in order to enable selection of the best treatment method. It is considered that tumor cells isolated from the tissue of the primary cancer lesion are closely associated with the metastasis of cancer.
In January 2009, jointly with Oncolys BioPharma, Inc., Sysmex developed a technology for detecting living tumor cells suspended in blood through use of a virus that replicates and emits fluorescence in tumor cells (TelomeScan® *). The technology, which is able to detect circulating tumor cells with high sensitivity, is expected to contribute to the development of a diagnostic method that is able to detect possible metastasis earlier and more accurately than the serum tumor marker test and diagnostic imaging (e.g. CT scan).
In cooperation with the Department of Breast and Endocrine Surgery, Osaka University Graduate School of Medicine, Faculty of Medicine, Department of Thoracic Surgery Kitasato University School of Medicine, and Department of Respiratory Medicine, Kitasato University School of Medicine, we have conducted joint research on breast and lung cancer. To date, it has been demonstrated that, by using the technology, circulated tumor cells can be detected in blood collected from patients with early and advanced cancer.
Sysmex has now initiated full-scale research on this technology in collaboration with the research group conducted by Dr. Tetsuya Nakatsura (Chief Researcher), Section Head, Section for Frontier Medicine, Project Leader, Cancer Immunotherapy Project, Investigative Treatment Division, Research Center for Innovative Oncology of National Cancer Center Hospital East. In this joint research, we will first examine if we are able to detect tumor cells in blood collected from patients with gastric, colon, esophageal, liver, and pancreatic cancer. Then, we will verify the clinical usefulness of the technology for each cancer type.
In order to accelerate the verification process, clinical doctors and a company researcher will cooperate in order for both sample processing and case studies to be conducted at the Clinical Development Center, National Cancer Center Hospital East.
We will also continue the process of verifying the technology's clinical usefulness for breast and lung cancer, working with the Department of Breast and Endocrine Surgery, Osaka University Graduate School of Medicine, Faculty of Medicine, Department of Thoracic Surgery, Kitasato University School of Medicine, and Department of Respiratory Medicine, Kitasato University School of Medicine.
| <Outline of the joint research program with the National Cancer Center Hospital East > | |
|
【Research Staff】
|
|
| 【Research Start Date】 February 15, 2010 | |
| 【Target Cancer Types】 Gastric cancer, colon cancer, esophageal cancer, liver cancer, and pancreatic cancer | |
| 【Measurement method of CTC detection technology】 | |
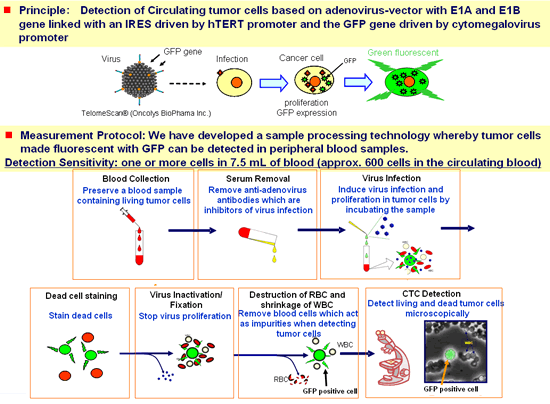
* Virus which proliferates in a living tumor cell developed by Oncolys BioPharma, Inc.
| (Reference) Ongoing joint research with other institutions | |
| 1) Joint research with the Department of Breast and Endocrine Surgery, Osaka University Graduate School of Medicine | |
| 【Research Staff】 Osaka University Graduate School: Principal investigator Dr. Shinzaburo Noguchi, Professor, Department of Breast and Endocrine Surgery Osaka Koseinenkin Hospital Dr. Fumine Tsukamoto, Department Head, Department of Breast and Endocrine Surgery |
|
| 【Research Start Date】 June 1, 2009 | |
| 【Target Cancer Type】 Breast cancer | |
| 2) Joint research with the Department of Thoracic Surgery and the Department of Respiratory Medicine, Kitasato University School of Medicine | |
| 【Research Staff】 Kitasato University School of Medicine: Principal Investigator Dr. Yukitoshi Sato, Professor, Department of Thoracic Surgery Dr. Noriyuki Masuda, Profesor, Department of Respiratory Medicine |
|
| 【Research Start Date】 July 15, 2008 | |
| 【Target Cancer Type】 Lung cancer | |
|
|
|
- Information contained in the news release is current as of the date of the announcement,
but may be subject to change without prior notice.
