
The Founding Era

Taking on the Challenge of the Medical Electronics Business
![]()
Taro Nakatani, then-vice president of leading megaphone manufacturer TOA ELECTRIC CO., LTD. (presently TOA Corporation), traveled to the United States in search of a new pillar of business.

Taro Nakatani setting off for his overseas tour
![]()
TOA ELECTRIC CO., LTD., decided to enter the medical electronics industry.
![]()

The automated hematology analyzer CC-1001
Successfully commercialized the automated hematology analyzer CC-1001, the first blood cell counter in Japan.
![]()
Founder Taro Nakatani announced the corporate attitude of “selling the results”
![]()
 MCC News, Volume 1
MCC News, Volume 1
Provision of scientific servicesCompany began publishing MCC News for hospital laboratories around Japan (MCC is an acronym for “micro cell counter.”)
![]()

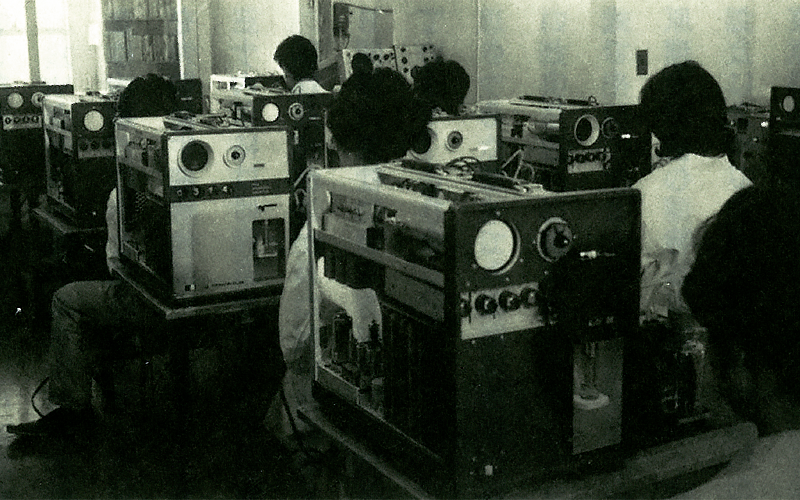
Members of the ME Equipment Division of then-TOA ELECTRIC CO., LTD.
TOA ELECTRIC CO., LTD., launched the ME Instrument Department(ME stands for “medical electronic instruments”)
![]()

The first reagents the Company developed: Cellkit, a diluent, and Saponin-S, a lysing reagent
Company began providing testing reagentsTo measure blood cells, both instruments and reagents are needed. To deliver accurate counting results to customers, even though it is an equipment manufacturer the Company made the decision to begin providing reagents.


The Birth of TOA Medical Electronics

Launching the Company and Strengthening Its Business Foundations
![]()

Members when TOA MEDICAL ELECTRONICS CO., LTD., was established
Establishment as a sales companyTOA MEDICAL ELECTRONICS CO., LTD. was founded to sell medical electronic equipment manufactured by TOA ELECTRIC CO., LTD. Taro Nakatani appointed first president.
![]()
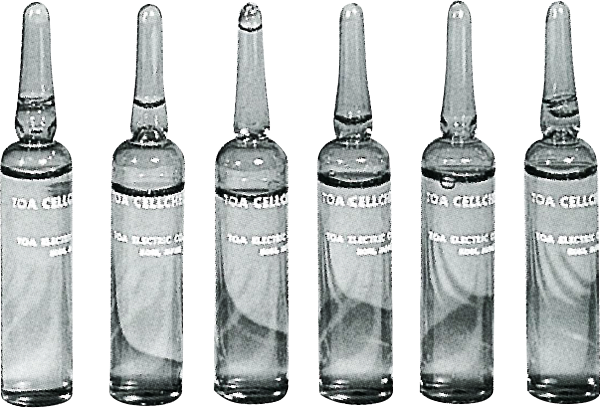
CELLCHECK-B
Company began providing standard reference substance for use in quality control, in the aim of increasing the reliability of counting resultsThe Company begins selling CELLCHECK-B, a standard reference substance that includes a defined number of blood cells, allowing laboratories to confirm the accuracy of their cell measurements.
Taro Nakatani advanced a new fundamental management policy, the “Three Aspects of Confidence”The Company formulated “Basics of Our Company Management,” summarizing fundamental management perspectives and action guidelines.

“Three Aspects of Confidence,” the fundamental management policy (current founding philosophy)
![]()

German representative office (Hamburg)
Establishment of first overseas location (Germany)
![]()

Kakogawa Factory (Kakogawa, Hyogo Prefecture)
Transition from a sales company to a specialized manufacturer of clinical testing instruments (integrated manufacturing and sales)The Company established the Kakogawa Factory and integrates the Sales, Development, Production and General Affairs divisions.
![]()
Proposal of stance outlining the pursuit of qualityTaro Nakatani addressed all employees, emphasizing that “Quality is our heart: the company's means to life.”

Employees deeply moved by Taro Nakatani’s fervent speech at the founding anniversary ceremony
![]()
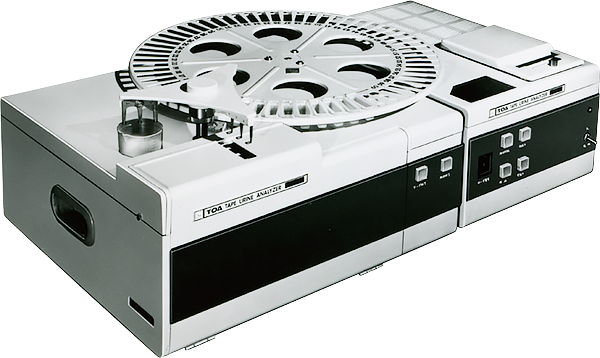
The simplified automated urinalysis analyzer TU-100
Entry into the urinalysis fieldThe Company launched the TU-100, a simplified automated urinalysis analyzer.
![]()

The automated hematology analyzer CC-710
Introduction of the CC-710, Japan’s first fully automated hematology analyzer
![]()

US representative office (San Francisco)
Start of entry into the world’s largest market (United States)


A Turning Point toward Growth

Launching the Sysmex Brand and Increasing Our Fields of Involvement
![]()

The birth of the Sysmex brandThe product brand transitions from TOA to Sysmex.
![]()

First TOA Hematology Seminar
Holding of the first hematology seminarThe Company put on a full-fledged seminar with the goal of “providing up-to-date information about hematology, and being of use around the world.”
Since that time, seminar themes have also expanded beyond hematology, and have continued as “scientific seminars.”
![]()

The fully automated hematology analyzer CC-720
Achievement of fully automated processing of hematology testsingThe Company launched the world’s first fully automatic blood cell counter featuring a sampler, the CC-720.
![]()

TOA MEDICAL ELECTRONICS(USA). INC.
Establishment of first overseas subsidiary (Los Angeles, United States)The Company established TOA MEDICAL ELECTRONICS (USA) INC. (presently Sysmex America, Inc.)
![]()

TOA MEDICAL ELECTRONICS (DEUTSCHLAND) GMBH
Establishment of European subsidiary (Hamburg, Germany)The Company established TOA MEDICAL ELECTRONICS (DEUTSCHLAND) GMBH (presently Sysmex Europe SE).
![]()

The automated hematology analyzer E-Series, a best-seller, with cumulative sales of more than 1,000 units
Use of “sheath flow” to enhance measurement precision and increase in the clinical value of counting results through three-part white blood cell differentiationThe Company launched the E-Series automated hematology analyzer.
![]()

The automated blood coagulation analyzer CA-100
Entry into the hemostasis fieldThe Company launched the CA-100, its first automated blood coagulation analyzer.
![]()

Kobe Factory (Nishi-ku, Kobe, Hyogo Prefecture)
Enhancement of R&D structure in the hemostasis, immunochemistry and urinalysis fieldsThe Company established the Kobe factory in the Seishin Industrial Park and relocates the R&D division (presently Technopark)
![]()

The automated immunochemical analyzer PAMIA-10, which uses the latex agglutination method to make measurements in 15 minutes
Entry into the immunochemistry fieldThe Company launched the PAMIA-10 immunochemical analyzer, which used a proprietary immunoassay method to measure tiny amounts of protein in serum.


The Transition to a Public Company

Strengthening Our Management Structure and Aiming to Be the Market Leader in Hematology
![]()
Reizo Hashimoto appointed president, stated “Within 10 years, we will become the global leader in the hematology field.” and “We aim for new business to account for more than 40% of sales.”
![]()

The automated reticulocyte analyzer R-1000
The Company launched the automated reticulocyte analyzer R-1000, the world’s first instrument to automatically measure reticulocytes.
![]()

The HS-Series, an integrated hematology system connecting the NE-Series, R-3000 and SP with a specialized belt conveyor
The Company launched the HS-Series integrated hematology system, the world’s first fully automated system handling blood cell counts, white blood cell differentiation, reticulocyte measurement and preparation of smear samples.

The automated urine sediment analyzer UA-1000
Entry into the sediment urinalysis field with products developed in-houseThe Company launched the automated urine sediment analyzer UA-1000.
![]()
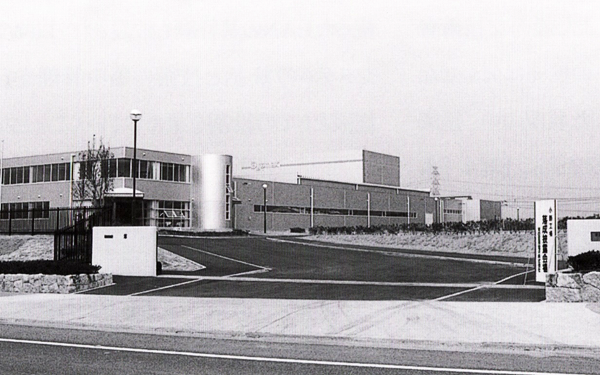
Ono Factory (Ono, Hyogo Prefecture)
Establishment of our first reagent factory in Japan (Ono, Hyogo Prefecture)

TOA MEDICAL ELECTRONICS (UK) LTD. (presently Sysmex UK Limited)
Commencement of direct sales, service and support for the first time overseas (United Kingdom)The Company established its first overseas sales subsidiary.
![]()

Neumünster Factory (Germany)
Start of reagent production overseasThe Company established a reagent production base in Europe.

SYSMEX CORPORATION OF AMERICA (presently Sysmex Reagents America, Inc. (United States))
Establishment of US reagent production base
![]()

Asia tour (1996)
Start of developing an operating base in the Asia Pacific regionThe Company established the Singapore representative office.
![]()

Accelerated business expansion in the hemostasis fieldThe Company concluded a business collaboration agreement with Dade International Inc. (presently Siemens Healthcare Diagnostics Inc.) regarding the sale of coagulation product lines.
![]()

Press conference at the launch of JINAN DONGYA MEDICAL ELECTRONICS CO. LTD
Establishment of a reagent supply and technical service structure in ChinaThe Company established a joint venture (presently Jinan Sysmex Medical Electronics Co., Ltd.) in Jinan, China.
![]()

The automated hematology analyzer SF-3000
Launch of the SF-3000 automated multiparameter hematology analyzer, combining a semiconductor laser and flow cytometry technologyThe Company promoted the spread of white cell differentiation instruments among small and medium-sized laboratories.

Shares listed on the Second Section of the Osaka Securities ExchangeThe Company Strengthened the management structure through various activities towards its listings. (listing on the Second Section of the Tokyo Stock Exchange in 1996 and the First Sections of the Tokyo and Osaka exchanges in 2000)
 The fully automated urine cell analyzer UF-100 which uses particle measurement technology to quantify elements in urine
The fully automated urine cell analyzer UF-100 which uses particle measurement technology to quantify elements in urine
Launch of the UF-100 fully automated analyzer of formed elements in urine, the world’s first fully automated instrument for sediment urinalysis testing


Toward Frontrunner Status
Leveraging Our Unique Strengths to Become the Global Leader
![]()
Hisashi Ietsugu (current Chairperson and Group CEO) appointed president and stated “We aim to transform the Company from a specialized manufacturer to a distinctive and global comprehensive manufacturer.”
 Change in president
Change in president
![]()
 Alliance with Roche
Alliance with Roche
Strengthening of the global sales network through business alliances with a leading global healthcare companyAlliance with F. Hoffman-La Roche Ltd. (Switzerland) in hematology products
Change of name to Sysmex Corporation, relocation of head office on the occasion of the 30th anniversary of establishment
 Sysmex Corporation’s head office (Chuo-ku, Kobe, Hyogo Prefecture)
Sysmex Corporation’s head office (Chuo-ku, Kobe, Hyogo Prefecture)
 SYSMEX SINGAPORE PTE LTD. (presently Sysmex Asia Pacific Pte Ltd.)
SYSMEX SINGAPORE PTE LTD. (presently Sysmex Asia Pacific Pte Ltd.)
Establishment of regional headquarters for the Asia Pacific region
Commencement of reagent production in IndiaThe Company established the joint venture SYSMEX TRANSASIA BIO-MEDICALS PRIVATE LTD. (presently Sysmex India Pvt. Ltd.).
![]()
 The automated hematology analyzer XE-2100
The automated hematology analyzer XE-2100
Launch of the XE-2100 automated multiparameter hematology analyzer, a culmination of technologies, achieving personnel savings and higher efficiencies in testing operationsIn 2007, the Company becomes the global leader in the hematology field.
 Customer support center
Customer support center
Enhancement of service quality through the online provision of external quality controlThe Company began providing Sysmex Network Communication Systems (SNCS), an online support service.
![]()
 Sysmex Shanghai Ltd.
Sysmex Shanghai Ltd.
Establishment of a regional headquarters in China
 Sysmex do Brasil Industria e Comercio Ltda. (Brazil)
Sysmex do Brasil Industria e Comercio Ltda. (Brazil)
Expansion of the global production system to ensure a stable supply of reagentsThe Company began producing reagents at subsidiaries in Brazil and Singapore.
![]()
 Central Research Laboratories (Nishi-ku, Kobe, Hyogo Prefecture)
Central Research Laboratories (Nishi-ku, Kobe, Hyogo Prefecture)
Beginning to take on the challenge of the life science fieldThe Company established the Central Research Laboratories at Techno Center (presently Technopark)
![]()

Reinforcement of R&D capabilities in the production of bio-diagnostic reagentsInternational Reagents Corporation became a wholly owned subsidiary. (Absorbed into Sysmex Corporation in 2022)
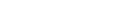
Toward the provision of total solutions in clinical testingThe Company launched a laboratory information system.
![]()

Commencement of direct sales and service in the United States(Sysmex America, Inc. was established)
![]()
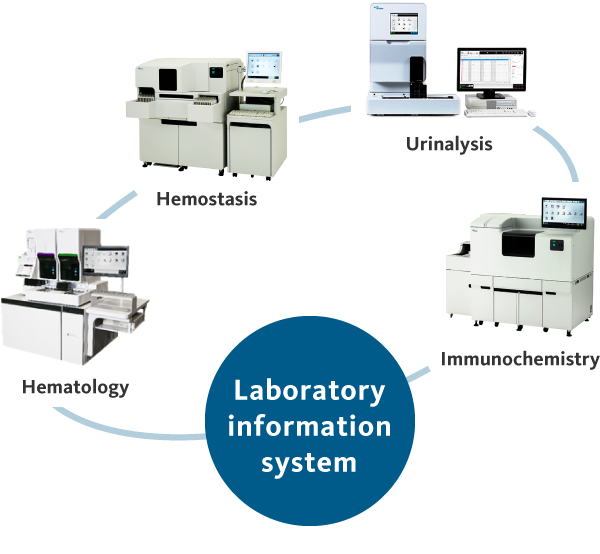
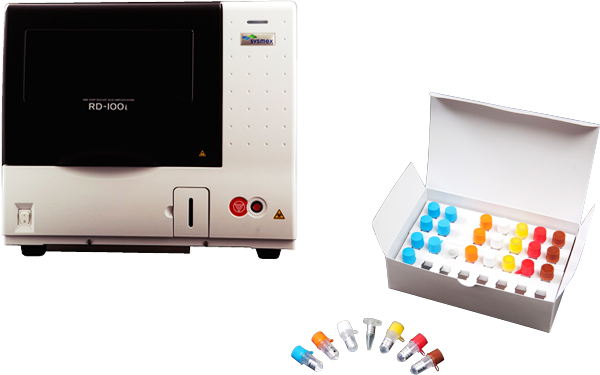 The gene amplification detector RD-100i and gene amplification reagent LYNOAMP BC
The gene amplification detector RD-100i and gene amplification reagent LYNOAMP BC
Contribution to improvements in patients’ quality of life through the realization of intraoperative rapid detection of lymph node metastasis of breast, colorectal and stomach cancersIn Europe, Sysmex launches its first product in the life science business: the RD-100i gene amplification detector and LYNOAMP BC, a related reagent.


Continuing to Design the Future
Taking on Challenges in the Advancement of Healthcare
![]()
Formulation of “Sysmex Way,” the corporate philosophy for the Sysmex Group

![]()
 The automated immunoassay system HISCL-2000i
The automated immunoassay system HISCL-2000i
Launch of the HISCL-2000i fully automated immunoassay system, realizing highly sensitive, high-speed testing of minute samples due to the development of the chemiluminescence enzyme immunoassay (CLEIA) method
![]()
 Corporate logo symbolizing Sysmex’s mission of “Shaping the advancement of healthcare.”
Corporate logo symbolizing Sysmex’s mission of “Shaping the advancement of healthcare.”
Updating of the corporate logo on the occasion of the 40th anniversary of establishment
Opening of Technopark, the Company’s core R&D base, under the concept of the “Creation of ‘knowledge’ and its inheritance”
 Technopark (Nishi-ku, Kobe, Hyogo Prefecture)
Technopark (Nishi-ku, Kobe, Hyogo Prefecture)
![]()
 The automated hematology analyzer XN-Series XN-9000
The automated hematology analyzer XN-Series XN-9000
Global launch of the XN-Series, our flagship model in the hematology field, aimed at establishing our position as the undisputed global leader in hematology
 The automated blood coagulation analyzer CS-5100
The automated blood coagulation analyzer CS-5100
Launch of the CS-5100 fully automated blood coagulation analyzer, a new model in the hemostasis field offering enhanced processing capacity
![]()
 The automated immunoassay system HISCL-5000
The automated immunoassay system HISCL-5000
Contribution to the realization of increased testing efficiency and pre-examination testing through fast measurement requiring only 17 minutes and advanced functionalityThe Company launched the HISCL-5000 fully automated immunoassay system, a new model in the immunochemistry testing field.
![]()
 Press conference at the launch of Medicaroid Corporation.
Press conference at the launch of Medicaroid Corporation.
Entry into the medical robotics marketSysmex jointly established Medicaroid Corporation with Kawasaki Heavy Industries, Ltd., as a marketing company toward the development of medical robots.

Acceleration of developing technology platforms toward personalized medicineSysmex acquired Partec GmbH (presently Sysmex Partec GmbH) of Germany, a pioneer in FCM technology.

Sysmex acquired Inostics GmbH (presently Sysmex Inostics GmbH), which possesses technology for the highly sensitive measurement of cancer genes in the blood.
![]()
 Lab-assay service for gene testing offered by RIKEN GENESIS Co., Ltd.
Lab-assay service for gene testing offered by RIKEN GENESIS Co., Ltd.
Entry into the lab-assay business for gene testingSysmex became an equity participant in RIKEN GENESIS Co., Ltd., to promote advances in the gene testing business.
![]()
Reconfiguration of instrument manufacturing system aimed at high “made-in-Japan” qualitySysmex opened i-Square, its core instrument factory, in Kakogawa, Hyogo Prefecture.
 i-Square (Kakogawa, Hyogo Prefecture)
i-Square (Kakogawa, Hyogo Prefecture)
![]()
 Fully Automated Urine Particle Analyzer UF-5000
Fully Automated Urine Particle Analyzer UF-5000
Sysmex launched UF-5000 fully automated analyzers of formed elements in urine, next-generation models in the field of sediment urinalysis testing.
![]()
 Oxford Gene Technology IP Limited has an expertise in the development of high-quality FISH probes and NGS reagent
Oxford Gene Technology IP Limited has an expertise in the development of high-quality FISH probes and NGS reagent
Expansion of portfolio in the life science businessSysmex acquired Oxford Gene Technology IP Limited, which possesses unique genetic analysis technologies.

