Sysmex’s Technologies
Gene Measurement Technologies
We are working on technologies to enable the measurement of gene quantities inside cancer cells and the detection of circulating cancer cell-derived DNA.
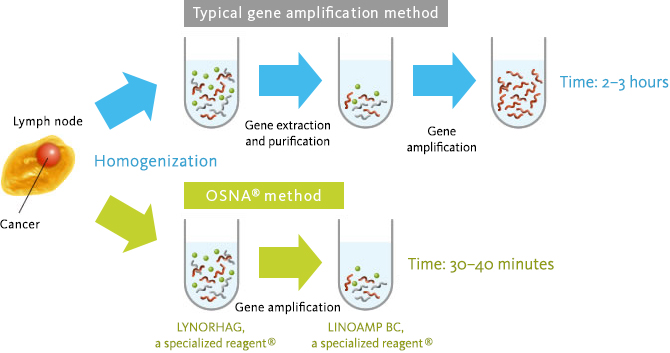
Sysmex Gene measurement technology : OSNA™
In general, gene amplification (such as the RT-PCR method) reaction requires a process to prevent impurities in the sample from affecting the gene amplification reaction. Also, it takes more than one hour for the gene amplification reaction.
Sysmex has developed the one-step measurement technology for cancer cell derived biomarkers (CK19mRNA) in the lymph node by optimizing composition of the pretreatment (homogenization) liquid and the raw materials (enzymes and primers) to enable elimination of process of the nucleic acid purification.
Current Initiatives in Gene Measurement Technologies
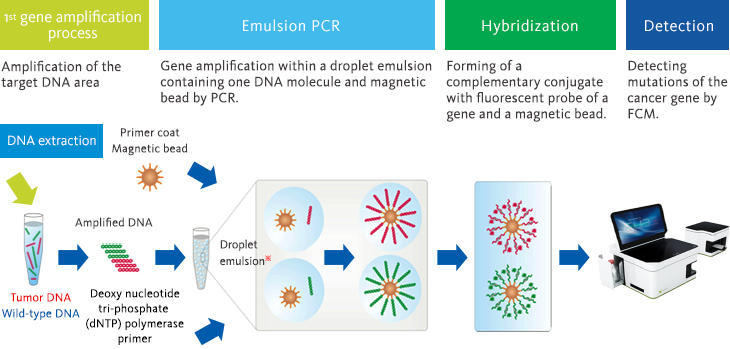
●BEAMing method
This technology contributes to the development of personalized medicine through the highly sensitive detection of cancer-related and other genes.
Using BEAMing method technology, we are creating a measurement system to detect cell-free DNA with a high degree of sensitivity and precision and working toward the realization of liquid biopsy.
BEAMing technology is used to capture individual DNA molecules with magnetic particles in droplets measuring several microns in diameter and then detecting the amplification of the DNA molecules on the magnetic particles.
This technology allows the detection of extremely small quantities (0.01%) of mutant genes present in wild-type genes.
Going forward, we will work to simplify and automate measurement so that it can be used in hospitals and commercial laboratories, thereby contributing to the development of personalized medicine.
●Plasma-Safe-SeqS
Very slight amounts of cancer-derived genes must be detected among huge amounts of normal genes in the blood in order to specify these mutations.
Next generation sequencers (NGS) are used to examine gene information, but they include a certain number of reading errors. Thus it can be difficult to distinguish an original gene mutation from a reading error of NGS when a mutation is detected.
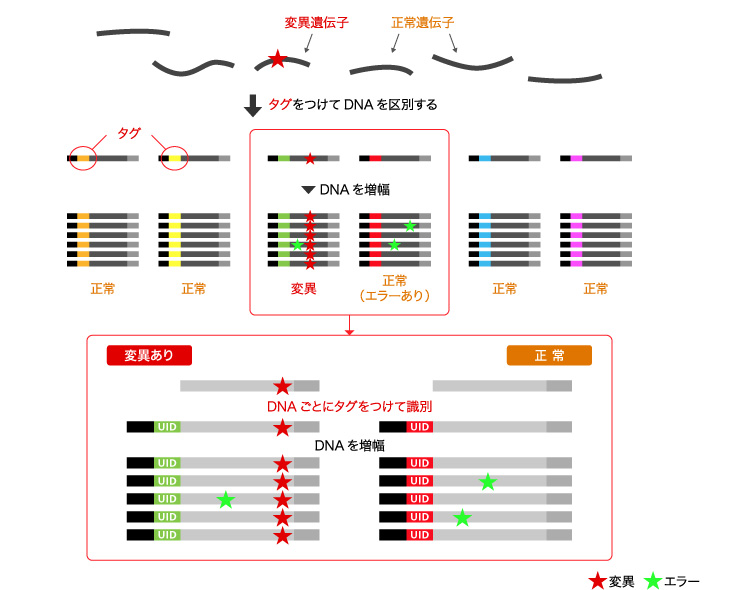
Sysmex is developing Plasma-Safe-SeqS (PSS) as a pretreatment technology to overcome NGS problems.
PSS technology differentiates gene mutations from reading errors by tagging amplified DNA. We detect slight amounts of original gene mutation to implement a liquid biopsy, and realize personalized medicine by contributing proper diagnostics and drug administration.
■Principle of Plasma-Safe-SeqS Technology
■Reference Animation of Plasma-Safe-SeqS
*SafeSEQ has changed its name to ctDNA analysis using Plasma-Safe-SeqS technology (RUO)
